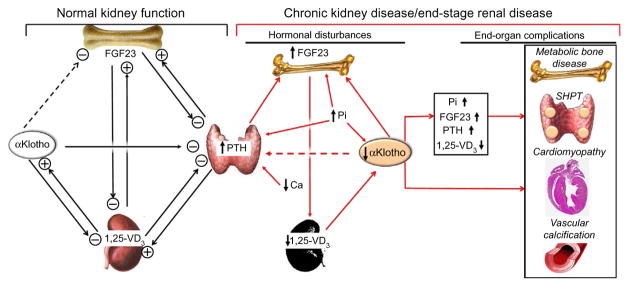
Fig. 3.
Proposed physiological role of αKlotho in mineral metabolism and pathophysiological consequences of αKlotho deficiency in CKD. In the setting of normal kidney function with normal αKlotho levels (left panel), αKlotho may suppress FGF23 production and release from the bone. But there is no direct evidence to prove it. αKlotho functions as coreceptor of FGFR to allow FGF23 to suppress PTH production and release from parathyroid gland. PTH stimulates and increases plasma levels of FGF23 and 1,25-(OH)2-vitamin D3. Increased 1,25-(OH)2-vitamin D3 further stimulates FGF23, and directly and indirectly suppresses PTH levels. Increased 1,25-(OH)2-vitamin D3 also stimulates αKlotho production in the kidney. Taken together, through several negative- or positive-feedback loops, αKlotho functions as both a phosphate and calcium regulatory hormone to directly or indirectly suppress PTH, 1,25-(OH)2-vitamin D3, and FGF23 production and release. αKlotho's action on the kidney is to prevent renal Pi retention and to prevent renal Ca loss. In CKD and ESRD (right panel), the network is deranged (red arrows). Renal αKlotho is decreased followed by decrease in plasma αKlotho. The downregulation of αKlotho increases FGF23 production via unknown mechanism, which in turn suppresses 1,25-(OH)2-vitamin D3 production in the kidney. Whether low plasma αKlotho renders parathyroid gland resistant to the suppressive effect of FGF23 on PTH production is not proven. However, decreased FGFR1/3 and αKlotho expression in the uremic parathyroid gland could make the gland resistant to FGF23, and triggers and/or promotes secondary hyperparathyroidism (SHPT). Low plasma Ca also participates in SHPT development. Hyperphosphatemia amplifies the high FGF23 and PTH levels, and low αKlotho levels in the blood. The high plasma PTH, Pi, and FGF23, and low plasma 1,25-(OH)2-vitamin D3 and αKlotho in concert contribute to the development of complications such as metabolic bone disease, SHPT, cardiomyopathy, and vascular calcification. Dash line: unproven putative roles of αKlotho. Ca, ion calcium; CKD, chronic kidney disease; ESRD, end-stage renal disease; FGFR, FGF receptor; Pi, phosphate; PTH, parathyroid hormone; SHPT, secondary hyperparathyroidism; 1,25-VD3, 1,25-(OH)2-vitamin D3.