Figure 7.
Association of Substrate-Bound OTULIN with LUBAC
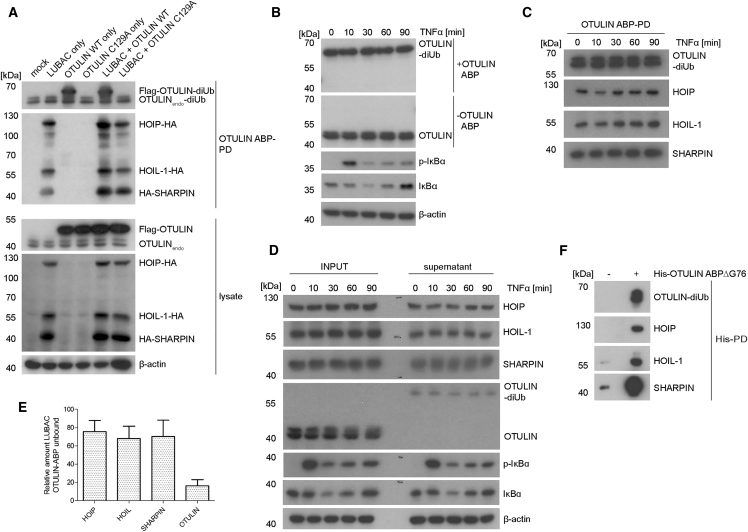
(A) HA-tagged LUBAC components and Flag-tagged OTULIN were co-transfected into HEK293 cells as indicated. Biotin-PD of OTULIN-diUb after OTULIN ABP incubation in cell extracts (∼1 × 107 cells) was analyzed for the association of transfected LUBAC proteins by western blot.
(B) Jurkat T cells were stimulated with TNF-α (20 ng/mL), and lysates (2.5 × 105 cells) were incubated with OTULIN ABP (1 μg) and analyzed for changes in OTULIN activity by western blot.
(C) Biotin-PD of OTULIN ABP was performed as in (A) from extracts of TNF-α-stimulated Jurkat T cells (2 × 107 cells, 4 μg OTULIN ABP). Interaction of endogenous OTULIN-diUb and LUBAC was assessed by western blot.
(D) Cell extracts of Jurkat T cells (+/− TNF-α stimulation) were subjected to OTULIN ABP incubation. OTULIN, HOIP, HOIL-1, and SHARPIN amounts were determined by western blotting prior (input) and after (supernatant) biotin-PD of OTULIN-diUb complexes.
(E) The majority of LUBAC subunits is not associated with OTULIN-diUb complexes. Biotin-PD of OTULIN-diUb complexes from extracts of unstimulated Jurkat T cells (2 × 107 cells) was performed as in (C). Levels of LUBAC subunits and OTULIN were quantified in the input and the supernatant. The quantified relative amounts of “free” OTULIN-diUb unbound proteins are depicted. Data represent the mean ± SD of six independent experiments.
(F) Extracts of Jurkat T cells (2 × 107 cells/sample) were treated with 4 μg OTULIN ABPΔG76 before His-PD. Binding of LUBAC components after PD was analyzed by western blot.