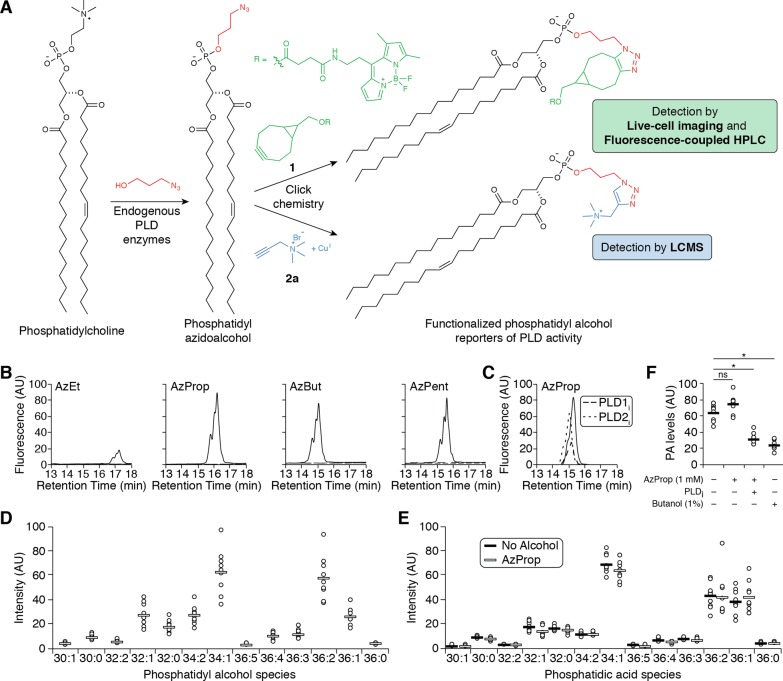
Figure 1.
Azidoalcohols can be used as reporters of PMA-stimulated, endogenous PLD activity in HeLa cells. (A) Schematic of method to monitor PLD activity using azidoalcohols and click chemistry. (B, C) Azidoalcohols can report on PLD activity in mammalian cells. HeLa cells were first treated with DMSO (solid lines) or a PLD inhibitor (B, FIPI (750 nM, dashed lines); C, VU0359595 (250 nM, dashed lines) or VU0364739 (350 nM, dotted lines)) for 30 min, then with the indicated azidoalcohol (1 mM) or vehicle (B, dotted lines) for 20 min, and then stimulated with PMA (100 nM) for 20 min, followed by lipid extraction, SPAAC tagging with BODIPY-cyclooctyne 1, and analysis by fluorescence-coupled HPLC. (D–F) AzProp faithfully reports on PLD-mediated PA synthesis and does not inhibit its production. (D, E) HeLa cells were labeled with AzProp (1 mM) for 20 min (D and E, white lines) or no alcohol (E, black lines) and then stimulated with PMA (100 nM) for 20 min, followed by lipid extraction, CuAAC labeling with alkynyl ammonium salt 2a, and analysis by LC/ESI-TOF MS. Shown are relative amounts of the individual phosphatidyl alcohol (D) or endogenous phosphatidic acid (E) species, indicated as number of carbons:degree of unsaturation in lipid tails. (F) HeLa cells were first treated with FIPI (PLDi, 750 nM) or DMSO vehicle for 30 min, then with AzProp (1 mM), butanol (1% w/v), or no alcohol for 20 min, and then stimulated with PMA (100 nM) for 20 min, followed by lipid extraction, CuAAC labeling with 2a and analysis by LC/ESI-TOF MS. Shown are the relative total amounts of all phosphatidic acid species detected. *, p < 0.01; ns, not significant. For D–F, n = 3 (3 technical replicates each of 3 independent biological experiments), and the horizontal bar represents the mean.