Abstract
Background
The fast pace of life, promoting fast food consumption and low physical activity, has resulted in obesity and/or diabetes as being serious social problems.
The aim of the present study was to evaluate concentrations of selected adipokines (leptin, adiponectin, resistin, and visfatin) and to assess the leptin/adiponectin ratio in plasma of type 2 diabetes (T2D) patients in relation to degree of obesity.
Material/Methods
The study comprised 92 T2D subjects divided into 4 groups according to BMI value – I (normal body weight), II (overweight), III (obesity), and IV (severe obesity) – and 20 healthy volunteers (control group). Each group was divided into male and female subgroups. Plasma concentrations of adipokines were determined by enzyme-linked immunosorbent assay.
Results
In women, leptin concentration was significantly higher in group IV, whereas in men it was higher in groups III and IV than in the control group and groups I and II. Irrespective of sex, a significant decrease in adiponectin level was observed in group III vs. control. There was no significant difference in resistin levels. In women visfatin was markedly enhanced in group III, whereas in men in groups II, III and IV vs. control. Leptin/adiponectin ratio was increased in groups III and IV vs. control in women, whereas in men vs. both control and group I.
Conclusions
The obese type 2 diabetic patients presented a disturbed adipokine profile, which seems to be an important link between obesity and T2D. The future studies concerning the question if regulating of adipokines’ concentrations could be a promising approach for managing metabolic disorders seem to be well-grounded.
MeSH Keywords: Adipokines; Diabetes Mellitus, Type 2; Obesity, Abdominal
Background
The fast pace of life, promoting consumption of high-calorie diet together with low physical activity, has resulted in obesity and/or diabetes as being serious social problems. World Health Organization (WHO) recognized both obesity and diabetes as epidemic diseases of the 21st century [1].
Obesity is a frequent concomitant of type 2 diabetes (T2D) [2]. It has been estimated that no less than 90% of type 2 diabetics are overweight or obese [3,4]. Obesity is regarded as one of the main risk factors of T2D development. It has been shown that lipid concentration increase in cytoplasm of adipocytes, myocytes, and hepatocytes, concomitant with obesity, is connected with development of insulin resistance in peripheral tissues [4–6]. Additionally, the common T2D therapies (e.g., sulphonyl urea derivatives, thiazolidinediones, and insulin) often cause body mass increase and subsequently intensify insulin resistance [3]. The risk of many co-existing diseases, particularly cardiovascular system disorders and chronic kidney diseases, is considerably increased in type 2 diabetics with concomitant obesity [7,8]. Curing such patients takes longer and is much less effective compared with subjects with normal body weight. Thus, T2D therapy is ineffective without parallel treatment of the concomitant obesity. Moreover, there is also evidence proving the efficacy of laparoscopic sleeve gastrectomy in the treatment of obese T2D patients [9].
The clarifying mechanisms connecting obesity with insulin resistance development, impaired glucose tolerance, and T2D is now the main target of scientific research [4]. It seems that the increased T2D risk in obese subjects can be, at least partially, explained by the changes in adipose tissue functions [10]. Apart from being the energy storage, the adipose tissue is also an important endocrine organ which secretes numerous substances with biological activity, including adipokines [4,11,12]. The literature shows that disturbances of adipokine secretion may contribute to peripheral insulin resistance development and/or impairment of production and action of insulin [2,13,14]. Pathophysiological relationships between obesity and T2D have not been fully clarified; nonetheless, adipokines (including leptin, adiponectin, resistin, and visfatin) seem to play an important role [2,14–16].
Leptin takes part in the long-term regulation of food intake; it causes appetite suppression as well as enhancement of energy expenditure [2,12]. Leptin decreases lipogenesis and stimulates lipolysis, as well as oxidation of fatty acids. Moreover, it is believed to exert a direct effect on glucose metabolism. However, chronic hyperleptinemia is thought to impair its physiological function [17].
The main physiological role of adiponectin is to increase insulin sensitivity [12,16]. Enhanced fatty acid oxidation in liver and skeletal muscles, as well as inhibition of hepatic glucose production, seem to be the primary mechanisms of such action [18]. This hypothesis seems to be confirmed by a recent study which showed that AMP-activated protein kinase (AMPK) impairment induced by adiponectin decrease resulted in insulin resistance in Bama mini-pigs fed a high-fat and high-sucrose diet [16]. Adiponectin has also been found to reduce production of TNFα – a cytokine thought to mediate insulin resistance [19].
Resistin has been proved to be a proinflammatory adipokine and its increase indirectly affects inflammation intensification. In light of the fact that diet-induced obesity leads to inflammation in adipose tissue and liver, it has been suggested that resistin, produced mainly by macrophages, contributes to insulin resistance [20].
Visfatin is thought to upregulate activity of NAD-dependent deacetylase sirtuin-1, an enzyme which influences insulin secretion in response to increase in glucose level [21].
The aim of the present study was to evaluate concentrations of selected adipokines – leptin, adiponectin, resistin, and visfatin – as well as leptin/adiponectin ratio, in plasma of T2D patients by degree of obesity.
Material and Methods
Study subjects
The study included patients suffering from type 2 diabetes hospitalized in the Diabetology Ward of Institute of Rural Health in Lublin (Poland) in the autumn and winter of 2012–2013 (to minimize effect of season on studied parameters). Ninety-two T2D patients (48 women and 44 men), aged 18–64 years were enrolled. The patients were all treated with oral blood glucose-lowering medication.
Insulin-independent diabetes was recognized based on the previous medical documentation confirming diagnosis or on the basis of the diagnostic process, including laboratory examinations according to criteria of the European Association for the Study of Diabetes as well as Polish Diabetes Association recommendations 2012.
The exclusion criteria were: insulin-dependent (type 1) diabetes, concomitant disturbances of liver and thyroid, renal insufficiency, chronic inflammatory diseases, age over 65, and excessive alcohol consumption.
The control group consisted of 20 healthy volunteers who were employees of the Medical University of Lublin (11 women and 9 men), aged 18–60 years. The absolute criterion of choice was normal BMI value (18.0–24.9 kg/m2) and lack of symptoms of inflammatory condition.
Anthropometric parameters such as age, body mass index (BMI), waist-hip ratio (WHR), systolic blood pressure (SBP), and diastolic blood pressure (DBP), as well as biochemical parameters such as total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and glycated hemoglobin (HbA1c), were determined for all the studied patients.
All subjects were informed about the aim of the experiment and gave written consent for participation in the study. The study protocol was approved by the Bioethical Board of Medical University of Lublin (Poland), acceptance KE-0254/100/2012.
Experimental design
The T2D subjects enrolled in the study were divided into 4 groups according to their BMI value: group I (normal body weight, 18.0–24.9 kg/m2); group II (overweight, 25.0–29.9 kg/m2); group III (obesity, 30.0–39.9 kg/m2); and group IV (severe obesity, >40.0 kg/m2). The control and T2D subjects were also subdivided depending on sex (W/M). The classification is presented in Table 1.
Table 1.
The general characteristics of controls and type 2 diabetics.
| Variable | Group K | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|---|
| Women | |||||
| No | 11 | 13 | 13 | 12 | 10 |
| Age | 49.00 [46.00–57.00] | 56.00 [53.00–64.00] | 56.00 [52.00–62.00] | 58.00 [55.00–61.00] | 55.00 [62.00–63.00] |
| BMI (kg/m2) | 22.90 [21.84–24.20] | 23.20 [21.93–24.39] | 27.73 [25.62–29.22] | 34.38***,C(K) [30.90–37.00] | 48.58***,C(K) [47.27–49.04] |
| WHR | 0.83±0.07 | 0.82±0.07 | 0.88±0.08 | 0.98±0.08***,C, X(H) | 0.91±0.05 |
| SBP (mmHg) | 120.00 [116.00–140.00] | 135.50 [116.50–152.50] | 150.50 [138.00–154.50] | 151.00 [135.00–161.50] | 155.00 [136.00–158.00] |
| DBP (mmHg) | 78.45±7.95 | 77.08±10.03 | 77.63±13.02 | 78.42±15.07 | 85.60±8.91 |
| HbA1c (%) | 4.58±0.43 | 8.62±1.90***(H) | 8.87±1.68***(H) | 9.28±1.46***(H) | 6.07±0.91X,#(H) |
| TC (mg/dl) | 183.82±36.52 | 186.31±36.46 | 185.22±45.47 | 178.50±52.43 | 158.20±13.37 |
| TG (mg/dl) | 101.00 [57.00–116.00] | 76.00 [59.00–115.00] | 84.00 [58.00–156.00] | 144.00 [127.00–181.00] | 103.50 [94.00–122.50] |
| HDL (mg/dl) | 68.27±12.25 | 69.15±12.82 | 70.22±22.83 | 52.60±15.43 | 54.80±9.73 |
| LDL (mg/dl) | 90.40±33.47 | 100.20±29.37 | 91.80±36.26 | 94.24±31.59 | 86.28±16.98 |
| Men | |||||
| No | 9 | 9 | 13 | 14 | 8 |
| Age | 36.00 [29.00–50.50] | 40.00 [32.00–52.00] | 43.00 [38.00–49.00] | 42.00 [39.00–64.00] | 41.00 [32.00–54.00] |
| BMI (kg/m2) | 21.89±2.02 | 21.46±2.48 | 26.89±1.42 | 34.47±3.15***,C(K) | 50.47±7.52***,C,Y(K) |
| WHR | 0.89 [0.87–0.92] | 0.92 [0.87–0.94] | 0.92 [0.90–0.99] | 1.04**(K) [1.02–1.06] | 1.10***,B,X(K) [1.04–1.19] |
| SBP (mmHg) | 122.88±18.62 | 133.67±40.99 | 137.67±17.04 | 147.29±28.66 | 148.17±30.71 |
| DBP (mmHg) | 85.00 [68.50–88.00] | 72.00 [54.00–100.00] | 80.50 [78.50–91.50] | 80.00 [79.00–90.00] | 90.50 [72.00–105.00] |
| HbA1c (%) | 4.38±0.50 | 8.90±2.22***(H) | 7.52±1.29***(H) | 7.92±1.73***(H) | 5.50±1.11B(H) |
| TC (mg/dl) | 199.00 [129.50–204.00] | 215.00 [149.00–228.00] | 163.00 [151.00–208.00] | 169.00 [155.00–189.00] | 185.50 [183.00–252.00] |
| TG (mg/dl) | 101.50 [87.50–104.00] | 102.00 [85.00–105.00] | 125.00 [82.00–139.00] | 204.00 [111.00–222.00] | 171.00 [103.00–229.00] |
| HDL (mg/dl) | 54.00 [35.00–63.50] | 57.00 [27.00–62.00] | 61.00 [50.00–67.00] | 40.50 [37.00–45.00] | 42.50 [40.00–47.00] |
| LDL (mg/dl) | 99.25±35.15 | 117.16±49.51 | 95.05±41.31 | 101.27±28.94 | 119.90±17.82 |
BMI – body mass index; WHR – waist-hip ratio; SBP – systolic blood pressure; DBP – diastolic blood pressure; TC – total cholesterol; TG – triglycerides; HDL – high-density lipoprotein; LDL – low-density lipoprotein. K – control group, group I – type 2 diabetic patients with normal body weight, group II – type 2 diabetic patients with overweight, group III – type 2 diabetic patients with obesity and group IV – type 2 diabetic patients with severe obesity.
p<0.01 vs. group K;
p<0.001 vs. group K;
p<0.01 vs. group I;
p<0.001 vs. group I;
p<0.05 vs. group II;
p<0.01 vs. group II;
p<0.05 vs. group III;
– Tukey’s post-hoc test;
– Kruskal-Wallis one way analysis of variance.
Normally distributed variables were expressed as mean ±SD, non-normally distributed ones as median and quartiles.
Biological material preparation
Vein blood samples were drawn from subjects after overnight fasting into heparinized test tubes. Plasma was separated by centrifugation (1000 g, 15 min.), divided into several portions (to avoid thawing-freezing cycle) and kept at −70°C for further examination. In the obtained material, concentrations of selected adipokines – leptin, adiponectin, resistin, and visfatin – were determined.
The determination of plasma concentrations of selected adipokines
The plasma concentrations of the studied adipokines were determined by immunoenzymatic methods using Human Leptin Quantikine ELISA Kit (R&D Systems, USA), Human Total Adiponectin/Acrp30 Quantikine ELISA (R&D Systems, USA), and Human Resistin Quantikine ELISA Kit (R&D Systems, USA), as well as Human Nicotinamide phosphoribosyltransferase, NAmPRTase ELISA Kit (Wuhan EIAab Science, China) for leptin, adiponectin, resistin, and visfatin, respectively. The concentrations are expressed in ng/ml for leptin, resistin, and visfatin, and in μg/ml for adiponectin.
Statistics
Statistical analysis was performed using StatSoft Statistica version 12.0 PL. Shapiro-Wilk test was used to verify conformity of variables distribution to hypothetical normal distribution. Leven’s test was used to evaluate homogeneity of variances. In case of variables distinguished by normal distribution and homogenous variances mean value as well as standard deviation were given. When lack of conformity to normal distribution or heterogeneous variances occurred, variables were described using median as well as quartile distribution [first quartile–third quartile]. For variables of normal distribution and homogenous variances difference significances were determined using a one-way analysis of variance (ANOVA) followed by Tukey HSD test, otherwise Kruskal-Wallis one-way analysis of variance by ranks together with multiple comparison post hoc test was applied. Values were considered significant with p<0.05.
Results
Anthropometric indices and biochemistry
Anthropometric indices such as age, BMI, WHR, SBP, and DBP, and biochemical parameters such as TC, LDL, HDL, and triglycerides, as well as HbA1c, were compared among groups. Irrespective of sex, a significant difference was obtained only in case of BMI, WHR, and HbA1c, as shown in Table 1.
Adipokines
Leptin
Leptin concentration was significantly higher in females (27.65 ng/ml [18.39–56.16] vs. 9.85 ng/ml [4.11–25.19], p<0.001).
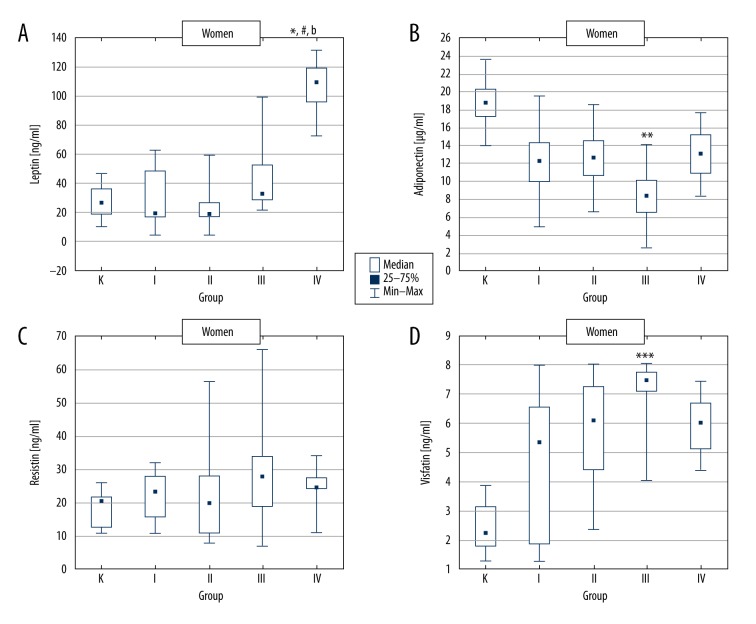
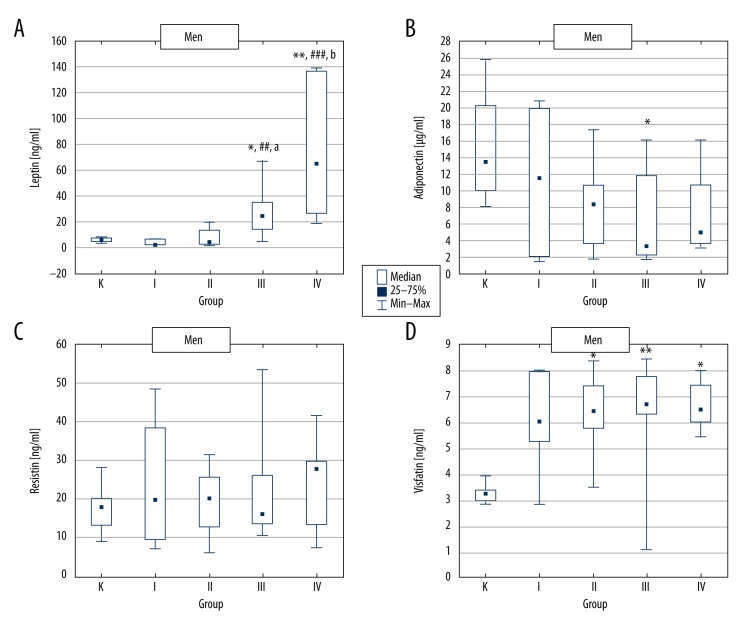
In women, plasma leptin concentration was significantly increased in group IV (T2D + severe obesity) and in men in groups III (T2D + obesity) and IV (T2D + severe obesity), as compared to respective control groups (Figures 1A, 2A).
Figure 1.
Adipokine concentrations in control group and type 2 diabetic women divided according to BMI value. K – control group, group I – type 2 diabetic patients with normal body weight, group II – type 2 diabetic patients with overweight, group III – type 2 diabetic patients with obesity and group IV – type 2 diabetic patients with severe obesity. * p<0.05 vs. group K; ** p<0.01 vs. group K; *** p<0.001 vs. group K; # p<0.05 vs. group I; b p<0.01 vs. group II.
Figure 2.
Adipokine concentrations in control group and type 2 diabetic men divided according to BMI value. K – control group, group I – type 2 diabetic patients with normal body weight, group II – type 2 diabetic patients with overweight, group III – type 2 diabetic patients with obesity and group IV – type 2 diabetic patients with severe obesity. * p<0.05 vs. group K; ** p<0.01 vs. group K; ## p<0.01 vs. group I; ### p<0.001 vs. group I; a p<0.05 vs. group II; b p<0.01 vs. group II.
The comparison of the results obtained for the T2D females revealed statistically significant plasma leptin increase in group IV vs. groups I and II (Figure 1A). In T2D males, leptin concentration was significantly increased in group III and IV compared to groups I and II (Figure 2A).
Adiponectin
Adiponectin level was significantly higher in females vs. males (13.20 μg/ml [8.13–8.53] vs. 8.29 μg/ml [3.17–14.08], p<0.01).
Both in women and men, a statistically significant decrease in plasma adiponectin concentration was observed in group III (T2D + obesity) vs. control. Additionally, in men, adiponectin was distinctly but insignificantly lower in group IV compared to control (Figures 1B, 2B).
Moreover, lower adiponectin concentrations in women from group III vs. groups I, II, and IV, as well as in men from groups III and IV vs. group I, were found. However, all these differences were insignificant (Figures 1B, 2B).
Resistin
There was no significant difference in resistin levels between women and men (22.80 ng/ml [14.81–28.06] vs. 18.08 ng/ml [13.28–26.22]), as well as in studied groups (Figures 1C, 2C).
Visfatin
Plasma visfatin concentration was not significantly different between women and men (5.40 ng/ml [3.00–7.26] vs. 6.33 ng/ml [4.61–7.42], respectively).
Irrespective of sex, in all T2D groups, plasma visfatin concentration was increased as compared to control. However, in women, this effect was significant only in group III (T2D + obesity). In contrast, in men, visfatin was markedly enhanced in groups II, III, and IV vs. control (Figures 1D, 2D).
The comparison of T2D groups showed slightly enhanced visfatin concentration in women from group III compared to the other diabetic groups. In T2D men, practically no differences were found (Figures 1D, 2D).
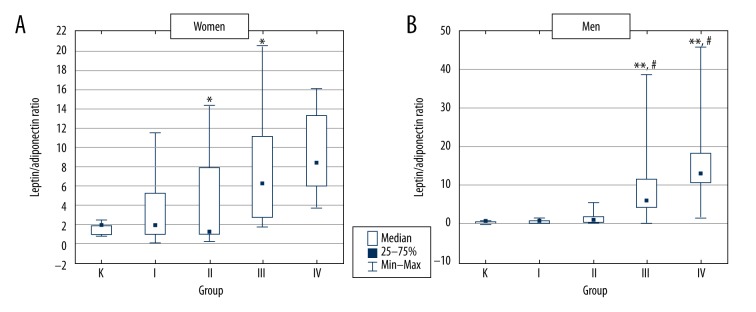
Leptin/adiponectin ratio
In women, leptin/adiponectin ratio was increased in all T2D groups compared to control, but differences reached statistical significance only in groups III and IV (Figure 3A). In men, leptin/adiponectin ratio was significantly higher in groups III and IV vs. both control and group I (Figure 3B).
Figure 3.
Leptin/adiponectin ratio in control group and type 2 diabetic patients divided according to BMI value. K – control group, group I – type 2 diabetic patients with normal body weight, group II – type 2 diabetic patients with overweight, group III – type 2 diabetic patients with obesity and group IV – type 2 diabetic patients with severe obesity. * p<0.05 vs. group K; ** p<0.01 vs. group K; # p<0.05 vs. group I.
Discussion
Leptin
In the current study, considerably increased plasma leptin level was found in women as compared to men. These results are consistent with findings reported by other authors [22–24]. It is believed to result from sexual dimorphism of adipose tissue distribution (in women it is most often accumulated subcutaneously and leptin is secreted in greater amounts by subcutaneous adipose tissue than the visceral one) as well as from positive influence of 17β-estradiol on leptin secretion [24]. Irrespective of sex, much higher leptin concentration was found in T2D obese subjects, with the highest values in the severe obesity group. These results were confirmed by other authors [11,25].
While the relationship between leptin and obesity seems to be clear, connections between plasma leptin concentration and T2D are not. Studies concerning leptin level in type 2 diabetics have revealed inconsistent outcomes [10,26,27], perhaps due to improper selection of study groups. Since sex and degree of obesity are believed to be the main factors determining leptin concentration [23,24], in the present study patients were divided into groups taking into consideration these factors. The slight decrease in leptin level observed by us in diabetic females and males with normal or enhanced body weight is consistent with the results obtained by Kazmi et al. [22]. This could be a consequence of relative insulin deficit observed in diabetics. In contrast, Rajković et al. [26] did not show any differences in leptin concentration between slim type 2 diabetics and slim healthy persons. Vasilescu et al. [28] reported no differences in leptin level between overweight diabetics and overweight healthy controls. The same observations were found in obese subjects with and without diabetes. On the contrary, Das et al. [29] reported significantly increased leptin concentration in T2D patients compared to healthy controls. However, it must be emphasized that they did not consider significant differences in BMI between the studied groups.
The presented outcomes suggest that the leptin increase observed in type 2 diabetics is dependent rather on concomitant obesity than diabetes per se. This assumption seems to be confirmed by the recent study involving obese T2D patients which showed that serum leptin level was reduced in the first year following biliopancreatic diversion, and remained substantially unchanged in the long-term in both the T2D remitter and non-remitter patients [25].
Adiponectin
We showed that the depletion of adiponectin concentration in all T2D patients, as well as its significant increase in females vs. males, revealed in the current study, is consistent with other authors’ reports [11,23,25,30]. Raghushaker et al. [6] showed significantly decreased adiponectin level in obese type 2 diabetics compared to healthy persons of normal weight. Neuparth et al. [31] displayed significant adiponectin decrease in diabetics noted by overweight and obesity compared to slim subjects, both diabetics and non-diabetics. Furthermore, while no difference between slim diabetics and non-diabetics was observed, adiponectin was significantly lower in obese diabetics than in overweight ones. These outcomes suggest that adiponectin level depends on obesity rather than on type 2 diabetes. In another study [15], the same authors showed that in individuals over age 65 years, adiponectin concentration depended strongly on concomitant obesity and T2D duration. Rajković et al. [26] found significantly lower adiponectin concentration in type 2 diabetics with normal body weight compared to weight-matched normoglycemic control. Moreover, adiponectin was found to be markedly decreased in overweight and obese diabetics compared to those with normal weight. However, overweight diabetics showed an only slightly lower adiponectin compared to obese ones. A similar effect was revealed in the present study comparing diabetic females with concomitant obesity to those with severe obesity. According to Rajković et al. [26], hypoadiponectinemia highly depends on type 2 diabetes, but, along with obesity, its concentration decreases much more rapidly. This seems to be confirmed by a recent study which showed a progressive significant increase of serum adiponectin level following biliopancreatic diversion only in T2D remitter patients, but not in non-remitter ones [25]. Additionally, Rajković et al. suggested that obesity influenced adiponectin only up to a certain threshold, above which further adiponectin decrease did not occur. This hypothesis seems to be rather daring and requires further confirmation [26]. It is probable that the slightly increased adiponectin concentration observed in the present study in severely obese women vs. obese ones results from the considerably lower percentage of glycated hemoglobin (HbA1c) as suggested in studies by Bhaktha et al. [32] and Tsai et al. [33], who reported the negative correlation between adiponectin and HbA1c percentage in type 2 diabetics. On the other hand, results presented by Rajković et al. [26] suggested the existence of a compensatory mechanism developed in obesity.
Resistin
No significant differences in resistin concentrations were shown in the present study. Similarly, Hansen et al. [34] did not find any differences in resistin level in obese and slim diabetics compared with slim healthy controls. Al-Suhaimi et al. [35] showed no differences between diabetics and non-diabetics or between normal weight healthy individuals and overweight/obese ones. The authors concluded that resistin was not involved in metabolic changes in insulin-independent diabetics. Similarly, Mohammadzadeh et al. [36] and Yaturu et al. [37] did not observe differences in resistin concentration between obese diabetics and non-diabetics. A recent study also showed only a slight difference between diabetic and non-diabetic subjects in relation to resistin [38].
On the contrary, Mabrouk et al. [39] showed significantly higher resistin concentration in obese diabetics vs. obese non-diabetics, as well as increased resistin level in obese diabetics and non-diabetics vs. slim healthy persons. In contrast, the current study revealed only slightly enhanced resistin level in severely obese diabetics vs. controls.
Visfatin
The present study showed no significant differences in visfatin concentration depending on sex, which confirms the observations of other authors [40,41]. The higher concentration of visfatin was found in all T2D patients (especially with concomitant obesity) compared to the control group. Similarly, Mabrouk et al. [39] found significantly higher levels of visfatin in obese type 2 diabetics compared to healthy normal weight ones and no differences between obese and slim diabetics. El-Shafey et al. [42] reported a significantly higher concentration of visfatin in obese diabetics compared to slim controls and no significant differences among slim type 2 diabetes and controls. However, unlike the present study, significantly higher visfatin levels in obese individuals with type 2 diabetes compared to diabetics with normal BMI was observed. On the other hand, a meta-analysis performed by Chang et al. [43] showed an elevated visfatin level in patients with T2D and confirmed a relationship between visfatin and glucose homeostasis, which was not affected by the extent of overweight/obesity. The study performed by Sheikh also revealed elevated visfatin level in overweight/obese T2D patients as compared to a weight-matched healthy group [38]. This is consistent with our results, as we found no influence of the degree of obesity on visfatin level. Moreover, in the current study, a slightly lower visfatin concentration in T2D women with severe obesity compared to obese ones was observed. As with adiponectin, this may result from the fact that the latter group had a much higher percentage of glycated hemoglobin, which is an indicator of long-term glycemic control. Shaker et al. [41] revealed a negative correlation between visfatin and the percentage of glycated hemoglobin. Interesting results were presented by Fioravanti et al. [44], who observed that after 3 weeks of weight loss therapy, visfatin concentrations were significantly reduced only in people with simple obesity, while in those with type 2 diabetes a slight increase was observed. The authors put forward the hypothesis that this resulted from a compensatory mechanism developed in response to impaired insulin action, which confirms insulinomimetic effect of visfatin. This theory seems to be confirmed by other studies [41,42,45], which demonstrated that plasma visfatin concentration was dependent on the degree of insulin resistance. However, it should be noted that the relationship between serum visfatin level and insulin resistance remains unclear and studies revealed conflicting results [41–43,45].
Leptin/adiponectin ratio
Since the leptin/adiponectin ratio is suggested to be a better determinant of insulin resistance and metabolic complications than leptin or adiponectin levels alone [11,46], we decided to examine this ratio in type 2 diabetic patients by the degree of obesity. In women, leptin/adiponectin ratio was significantly higher in obese T2D patients compared to controls, whereas in men it was higher compared to both controls and diabetics with normal body weight. In the literature data, there are only few studies investigating this relationship but their results are similar to ours. Chearskul et al. [47] showed higher ratio of leptin/adiponectin in obese adults with T2D compared to non-obese ones. Al-Hamodi et al. [10] in turn found significantly elevated leptin/adiponectin ratio in both obese subjects and non-obese type 2 diabetic patients vs. healthy group. In the recent study, it was shown that leptin/adiponectin ratio was associated with each of the components of the metabolic syndrome in child survivors of lymphoma and acute lymphoblastic leukemia [48].
Conclusions
Obese type 2 diabetic patients presented a disturbed adipokine profile in the form of significantly increased leptin and visfatin levels and decreased adiponectin concentration in comparison with the healthy controls.
As plasma leptin and adiponectin concentrations were not affected by diabetes per se, this could show that their alterations may be an important link between obesity, insulin resistance, and T2D. However, it is difficult to decide explicitly if hypoadiponectinemia is dependent on a higher degree on type 2 diabetes and is obesity intensifies this effect, or if it is the opposite.
The incidence of type 2 diabetes seems to be connected with an increase in visfatin, and the coexistence of obesity appears to intensify this effect.
Further research is needed on whether regulating of adipokines, especially leptin, adiponectin, and visfatin concentrations, in T2D and obese patients could be a promising novel approach for managing metabolic disorders.
Footnotes
Source of support: Departmental sources
References
- 1.World Health Organization. http://www.who.int/research/en/
- 2.Qadir MI, Ahmed Z. lep Expression and its role in obesity and type-2 diabetes. Crit Rev Eukaryot Gene Expr. 2017;27(1):47–51. doi: 10.1615/CritRevEukaryotGeneExpr.2017019386. [DOI] [PubMed] [Google Scholar]
- 3.Niu G, Li J, Wang H, et al. Associations of A-FABP with anthropometric and metabolic indices and inflammatory cytokines in obese patients with newly diagnosed type 2 diabetes. Biomed Res Int. 2016;2016:9382092. doi: 10.1155/2016/9382092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urbanavičius V, Abalikšta T, Brimas G, et al. Comparison of changes in blood glucose, insulin resistance indices, and adipokine levels in diabetic and nondiabetic subjects with morbid obesity after laparoscopic adjustable gastric banding. Medicina (Kaunas) 2013;49(1):9–14. [PubMed] [Google Scholar]
- 5.Etemad A, Ramachandran V, Pishva SR, et al. Analysis of Gln223Agr polymorphism of Leptin Receptor Gene in type II diabetic mellitus subjects among Malaysians. Int J Mol Sci. 2013;14(9):19230–44. doi: 10.3390/ijms140919230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghushaker CR, Shashank T, Manista A, et al. Evaluation of serum bio markers [Leptin, Adiponectin, Crp, Oxidised- Ldl] and lipid parameter in obese with type-2 diabetes mellitus: Risk to CVD. JARBS. 2013;5(3):250–53. [Google Scholar]
- 7.Berk KA, Vongpromek R, Jiang M, et al. Levels of the soluble LDL receptor-relative LR11 decrease in overweight individuals with type 2 diabetes upon diet-induced weight loss. Atherosclerosis. 2016;254:67–72. doi: 10.1016/j.atherosclerosis.2016.09.066. [DOI] [PubMed] [Google Scholar]
- 8.Stępień M, Stępień A, Wlazeł RN, et al. Obesity indices and adipokines in non-diabetic obese patients with early stages of chronic kidney disease. Med Sci Monit. 2013;19:1063–72. doi: 10.12659/MSM.889390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y, Xu X, Wu A, et al. [Effect of laparoscopic sleeve gatrectomy on type 2 diabetes mellitus in obese patients with body mass index less than 40 kg/m2]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20(4):400–4. [in Chinese] [PubMed] [Google Scholar]
- 10.Al-Hamodi Z, Al-Habori M, Al-Meeri A, Saif-Ali R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol Metab Syndr. 2014;6(1):99. doi: 10.1186/1758-5996-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picu A, Petcu L, Ştefan S, et al. Markers of oxidative stress and antioxidant defense in romanian patients with type 2 diabetes mellitus and obesity. Molecules. 2017;22(5):E714. doi: 10.3390/molecules22050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepien M, Rosniak-Bak K, Paradowski M, et al. Waist circumference, ghrelin and selected adipose tissue-derived adipokines as predictors of insulin resistance in obese patients: Preliminary results. Med Sci Monit. 2011;17(11):PR13–18. doi: 10.12659/MSM.882030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Højbjerre L, Sonne MP, Alibegovic AC, et al. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34(10):2265–72. doi: 10.2337/dc11-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bełtowski J. Apelin and visfatin: Unique “beneficial” adipokines upregulated in obesity? Med Sci Monit. 2006;12(6):112–19. [PubMed] [Google Scholar]
- 15.Coimbra S, Brandão Proença J, Santos-Silva A, Neuparth MJ. Adiponectin, leptin, and chemerin in elderly patients with type 2 diabetes mellitus: A close linkage with obesity and length of the disease. Biomed Res Int. 2014;2014:701915. doi: 10.1155/2014/701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu M, Xiang L, Liu Y, et al. Adiponectin induced AMPK impairment mediates insulin resistance in bama mini-pig fed high-fat and high-sucrose diet. Asian-Australas J Anim Sci. 2017 doi: 10.5713/ajas.17.0006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinehr T, Woelfle J, Wiegand S, et al. Leptin but not adiponectin is related to type 2 diabetes mellitus in obese adolescents. Pediatr Diabetes. 2016;17(4):281–88. doi: 10.1111/pedi.12276. [DOI] [PubMed] [Google Scholar]
- 18.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Leal Vde O, Mafra D. Adipokines in obesity. Clin Chim Acta. 2013;419:87–94. doi: 10.1016/j.cca.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Park HK, Ahima RS. Resistin in rodents and humans. Diabetes Metab J. 2013;37:404–14. doi: 10.4093/dmj.2013.37.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlová T, Novák J, Bienertová-Vašků J. The role of visfatin (PBEF/Nampt) in pregnancy complications. J Reprod Immunol. 2015;112:102–10. doi: 10.1016/j.jri.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kazmi A, Tariq KM, Hashim R. Association of leptin with type 2 diabetes in non-obese subjects. J Ayub Med Coll Abbottabad. 2012;24:186–89. [PubMed] [Google Scholar]
- 23.Susilowati R, Sulistyoningrum DC, Witari NP, et al. Sexual dimorphism in interleukin 17A and adipocytokines and their association with insulin resistance among obese adolescents in Yogyakarta, Indonesia. Asia Pac J Clin Nutr. 2016;25(1):93–S101. doi: 10.6133/apjcn.122016.s13. [DOI] [PubMed] [Google Scholar]
- 24.Mohammadzadeh G, Zarghami N. Serum leptin level is reduced in non-obese subjects with type 2 diabetes. Int J Endocrinol Metab. 2013;11(1):3–10. doi: 10.5812/ijem.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adami GF, Gradaschi R, Andraghetti G, et al. Serum leptin and adiponectin concentration in type 2 diabetes patients in the short and long term following biliopancreatic diversion. Obes Surg. 2016;26(10):2442–48. doi: 10.1007/s11695-016-2126-z. [DOI] [PubMed] [Google Scholar]
- 26.Rajković N, Zamaklar M, Lalic K, et al. Relationship between obesity, adipocytokines and inflammatory markers in type 2 diabetes: Relevance for cardiovascular risk prevention. Int J Environ Res Public Health. 2014;11(4):4049–65. doi: 10.3390/ijerph110404049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu X, Chen Z, El Bayoumy I. Serum leptin levels in obese women with and without type 2 diabetes mellitus. Minerva Endocrinol. 2014;39(3):223–29. [PubMed] [Google Scholar]
- 28.Vasilescu R, Ifrim S, Ionescu-Tirgoviste C. Relationship between plasma adipokines, inflammation, insulin resistance and subclinical atherosclerosis in newly diagnosed type 2 diabetes. J Diab Mell. 2011;1(2):17–25. [Google Scholar]
- 29.Das P, Bhattacharjee D, Bandyopadhyay SK, et al. Association of obesity and leptin with insulin resistance in type 2 diabetes mellitus in Indian population. Indian J Physiol Pharmacol. 2013;57(1):45–50. [PubMed] [Google Scholar]
- 30.Mohammadzadeh G, Ghaffari MA. Additional effect of diabetes mellitus type 2 on the risk of coronary artery disease: Role of serum adiponectin. Iran Red Crescent Med J. 2014;16(1):e8742. doi: 10.5812/ircmj.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuparth MJ, Proença JB, Santos-Silva A, Coimbra S. Adipokines, oxidized low-density lipoprotein, and C-reactive protein levels in lean, overweight, and obese portuguese patients with type 2 diabetes. ISRN Obes. 2013;2013:142097. doi: 10.1155/2013/142097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhaktha G, Nayak B, Mayya S, Shantaram M. Adiponectin levels in diabetic subjects without any pre-existing micro and macro vascular complications. Asian J Biochem Pharmaceut Res. 2014;4(2):150–57. [Google Scholar]
- 33.Tsai CJ, Hsieh CJ, Tung SC, et al. Acute blood glucose fluctuations can decrease blood glutathione and adiponectin levels in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;98(2):257–63. doi: 10.1016/j.diabres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Hansen D, Dendale P, Beelen M, et al. Plasma adipokine and inflammatory marker concentrations are altered in obese, as opposed to non-obese, type 2 diabetes patients. Eur J Appl Physiol. 2010;109(3):397–404. doi: 10.1007/s00421-010-1362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AL-Suhaimi EA, AL-Kulaifi F, Shehzad A. Serum adipokines metabolic function and immunological correlations in treated type 2 diabetes mellitus and obese subjects. World Applied Sciences Journal. 2013;22(7):933–38. [Google Scholar]
- 36.Mohammadzadeh G, Zarghami N, Mobaseri M. Serum resistin concentration in obese diabetic patients: Any possible relation to insulin resistance indices? Int J Endocrinol Metab. 2008;4:183–93. [Google Scholar]
- 37.Yaturu S, Daberry RP, Rains J, Jain S. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34:219–23. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Al Sheikh MH. The determinants of leptin levels in diabetic and nondiabetic Saudi Males. Int J Endocrinol. 2017;2017:3506871. doi: 10.1155/2017/3506871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mabrouk R, Ghareeb H, Shehab A, et al. Serum visfatin, resistin and IL-18 in A group of Egyptian obese diabetic and non diabetic individuals. Egypt J Immunol. 2013;20(1):1–11. [PubMed] [Google Scholar]
- 40.Mazaherioun M, Hosseinzadeh-Attar MJ, Janani L, et al. Elevated serum visfatin levels in patients with acute myocardial infarction. Arch Iran Med. 2012;15(11):688–92. [PubMed] [Google Scholar]
- 41.Shaker O, El-Shehaby A, Zakaria A, et al. Plasma visfatin and retinol binding protein-4 levels in patients with type 2 diabetes mellitus and their relationship to adiposity and fatty liver. Clin Biochem. 2011;44:1457–63. doi: 10.1016/j.clinbiochem.2011.08.1148. [DOI] [PubMed] [Google Scholar]
- 42.El-Shafey EM, El-Naggar GF, Al-Bedewy MM, El-Sorogy H. Is there a relationship between visfatin level and type 2 diabetes mellitus in obese and non obese patients. J Diabetes Metab. 2012:11. S. [Google Scholar]
- 43.Chang YH, Chang DM, Lin KC, et al. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27(6):515–27. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 44.Fioravanti A, Adamczyk P, Pascarelli NA, et al. Clinical and biochemical effects of a 3-week program of diet combined with spa therapy in obese and diabetic patients: A pilot open study. Int J Biometeorol. 2015;59(7):783–89. doi: 10.1007/s00484-014-0894-5. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Q, Dong W, Qian L, et al. Visfatin inhibits apoptosis of pancreatic β-cell line, MIN6, via the mitogen-activated protein kinase/phosphoinositide 3-kinase pathway. J Mol Endocrinol. 2011;47(1):13–21. doi: 10.1530/JME-10-0106. [DOI] [PubMed] [Google Scholar]
- 46.Dullaart RP, Gruppen EG, Connelly MA, et al. GlycA, a biomarker of inflammatory glycoproteins, is more closely related to the leptin/adiponectin ratio than to glucose tolerance status. Clin Biochem. 2015;48(12):811–14. doi: 10.1016/j.clinbiochem.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Chearskul S, Sriwijitkamol A, Kooptiwut S, et al. Cardiometabolic risk in Thai adults with type 2 diabetes mellitus: obese versus non-obese. J Med Assoc Thai. 2015;98(6):528–34. [PubMed] [Google Scholar]
- 48.Barbosa-Cortés L, López-Alarcón M, Mejía-Aranguré JM, et al. Adipokines, insulin resistance, and adiposity as a predictors of metabolic syndrome in child survivors of lymphoma and acute lymphoblastic leukemia of a developing countr. BMC Cancer. 2017;17:125. doi: 10.1186/s12885-017-3097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]