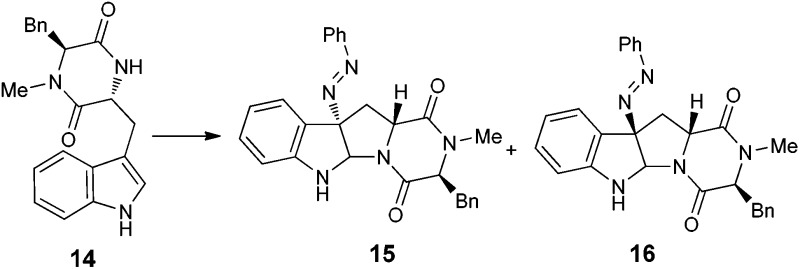
Table 1. Diastereoselective azo-coupling of tryptophan derivatives a .
| |||||
| Entry | Solvent | T (°C) | Reaction time | 15 : 16 | Yield b (%) |
| 1 | MeOH | –78 | 1 h | 1 : 1 | 66% |
| 2 | MeCN | rt | 24 h | — | — c |
| 3 | Toluene | rt | 24 h | 1 : 1.5 | 41% |
| 4 | THF | rt | 24 h | 1 : 1.5 | 74% |
| 5 | THF | 15 | 24 h | Only 16 | 14% |
| 6 | DCE | 15 | 24 h | 1 : 5 | 82% |
| 7 | DCE | rt | 24 h | 1 : 5 | 57% |
| 8 | DCE–THF(1 : 1) | 15 | 48 h | 1 : 10 | 63% |
| 9 | DCE–THF(1.5 : 1) | 15 | 48 h | <1 : 25 | 67% (61 d %) |
| 10 | DCE–THF(2 : 1) | 15 | 96 h | 1 : 20 | 50% |
| 11 | DCE–THF(4 : 1) | 15 | 96 h | 1 : 4 | 43% |
aConditions: 14 (0.1 mmol), PhN2BF4 (0.11 mmol), K2CO3 (0.15 mmol) and solvent (1 mL).
bNMR yield with 1,3,5-trimethoxylbenzene as internal standard.
cNo desired product was detected.
dIsolated yield.
