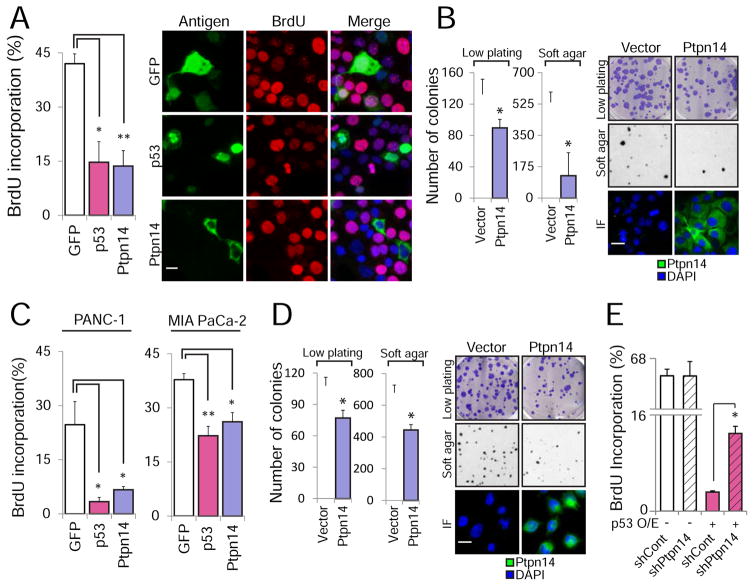
Figure 4. Ptpn14 overexpression drives growth arrest.
(A) The effect of HA-GFP, HA-Ptpn14 or HA-p53 expression on cell cycle progression in Kras+/G12D;Pdx1-Cre;Trp53fl/fl mouse PDAC cells was examined by BrdU immunostaining cells expressing each antigen (detected by GFP or HA immunofluorescence). (Left) The average BrdU incorporation ± SD from (n=3), with 100 cells counted per experiment, is shown. (Right) Representative images are shown; arrows point to BrdU+ cells, while arrowheads point to BrdU− cells. (B) (Left) Average colony number ± SD of KPC cells infected with an empty vector or a HA-Ptpn14 vector for low plating and soft agar assays (n=2, triplicate). (Right) Representative images of crystal violet-stained low plating (2 weeks after seeding), Giemsa-stained soft agar (4 weeks after seeding) and Ptpn14 localization by immunofluorescence. (C) The effect of HA-GFP, HA-Ptpn14 or HA-p53 expression on cell cycle progression in PANC-1 and MIA PaCa-2 human PDAC cells was examined by BrdU immunostaining. The average BrdU incorporation ± SD, with 100 cells counted per experiment, is shown (n=3). (D) (Left) Average colony number ± SD of MIA PaCa-2 cells infected with an empty vector or a HA-Ptpn14 vector for both low plating and soft agar assays (n=2, triplicate). (Right) Representative images of crystal violet-stained low plating (2 weeks after seeding), Giemsa-stained soft agar (4 weeks after seeding) and Ptpn14 localization by immunofluorescence. (E) The effect of HA-p53 expression on cell cycle progression in KPC PDAC cells expressing luciferase control shRNA (shCont) or shPtpn14-2 was examined by BrdU immunostaining. The average BrdU incorporation ± SD was assessed, with 100 cells counted per experiment (n=3). * p ≤ 0.05; ** p ≤ 0.001, two-tailed unpaired Student’s t-test. The scale bar in each panel applies to all images in that panel.