FIGURE 1.
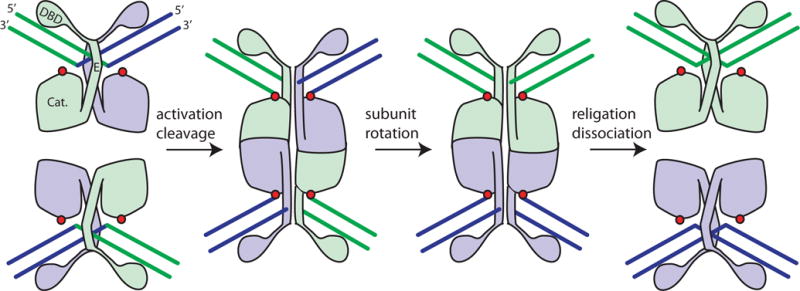
Cartoon of serine resolvase-mediated strand exchange. The wild-type protein initially binds crossover sites as an inactive dimer. Upon activation (see text), the catalytic domains (labeled “Cat.”) form a tetramer that synapses the two partner sites. Within the tetramer, the active site serines (red dots) attack the DNA, creating double strand breaks with 5′ phosphoserine linkages and 2-nucleotide 3′ overhangs. Two subunits and the DNA segments covalently linked to them can then rotate relative to the other two. A 180° rotation aligns the broken ends for re-ligation in the recombinant configuration. Much of both the dimer and tetramer interface is contributed by helix E. doi:10.1128/microbiolspec.MDNA3-0045-2014.f1
