Figure 1. Identification of RAB11a and RAB27b as binding partners for exocyst complex and their co-association with intracellular UPEC.
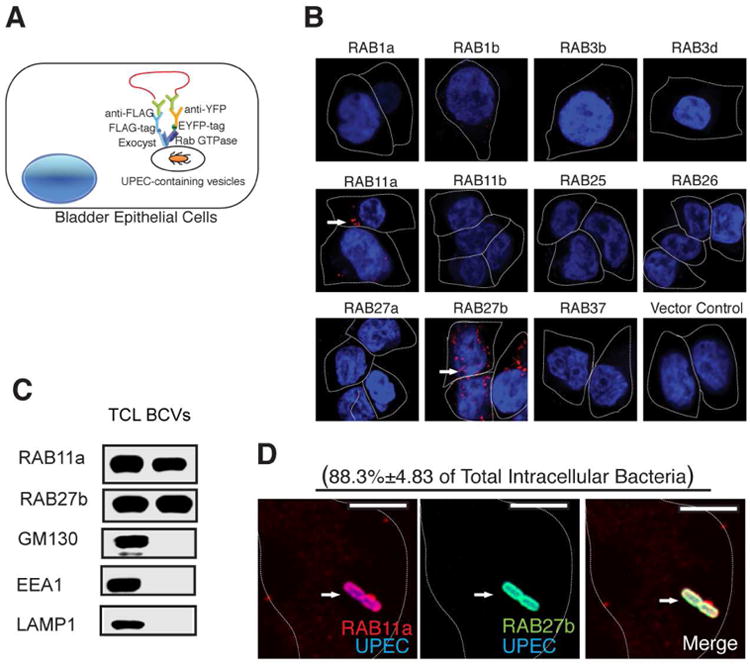
(A) Diagram showing PLA based assay for the identification of Rab proteins in BECs that bind Exocyst complex upon UPEC infection. 5637 BECs were transfected with lentivirus carrying various Rab-EYFP, as well as FLAG tagged exocyst complex components. The cells were then infected with UPEC, fixed and incubated with primary antibodies against EYFP or FLAG followed by Duolink in situ PLA probe-linked secondary antibodies. After ligation, the signals were amplified and detected by confocal microscopy.
(B) Proximity Ligation Assay detecting RAB11a and RAB27b (signals are indicated by an arrow) as binding partners for the Exocyst complex upon UPEC infection.
(C) Detection of RAB11a and RAB27b in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from BECs infected with UPEC by western blot. Unrelated organelle markers (GM130 for Golgi, EEA1 for early endosome, LAMP1 for late endosome and lysosome) were used to confirm the purity of the isolated BCV fraction.
(D) Immunofluorescence staining of infected BECs revealing the co-association of RAB27b (green) and RAB11a (red) with UPEC (blue). The percentage of intracellular bacteria that stain positive for RAB11a and RAB27b (double positive) over total intracellular UPEC counted (approximately 50 bacteria on each slide) is indicated in parenthesis. n=3 slides Scale bar: 5 μm. See also Figure SI
