Figure 5. Exocyst complex form a molecular bridge linking RAB11a and RAB27b-associated trafficking circuits.
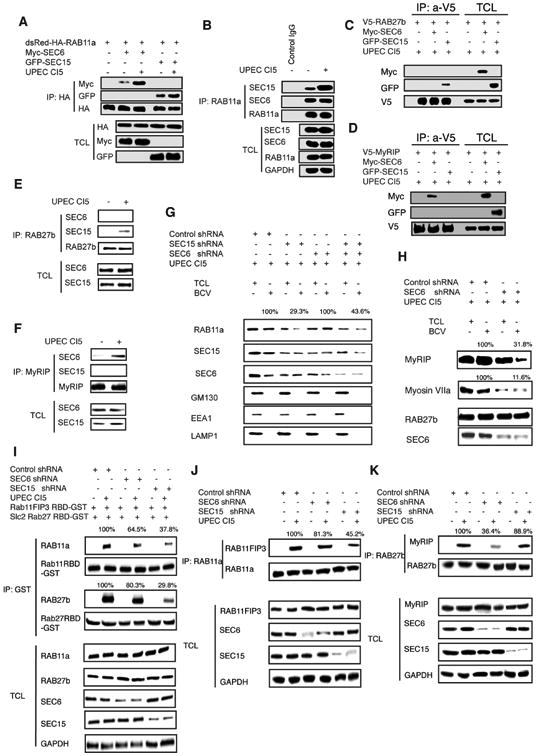
(A) HA tagged WT RAB11a immunoprecipitated from uninfected or infected BECs. The association between RAB11a and Exocyst complex protein SEC6 or SEC15 in different conditions was examined by western blots of the IP fractions. The proteins of interests in the total cell lysate (TCL) was depicted to indicate that similar levels of RAB11a, SEC6 or SEC15 protein were present in each fraction.
(B) Endogenous RAB11a immunoprecipitated from uninfected or infected BECs. The association between RAB11a and endogenous Exocyst complex protein SEC6 or SEC15 in different conditions was examined by western blotting of the IP fractions. The proteins of interests in the total cell lysates (TCL) was also depicted to indicate that similar levels of RAB11a, SEC6 or SEC15 protein was present in each fraction.
(C) V5 tagged WT RAB27b immunoprecipitated from infected BECs. The association between RAB27b and Exocyst complex protein SEC6 or SEC15 was examined by western blotting of these IP fractions. The protein of interests in the total cell lysates (TCL) was depicted to demonstrate that similar levels of RAB27b protein was present in each fraction.
(D) V5 tagged MyRIP immunoprecipitated from infected BECs. The association between MyRIP and Exocyst complex protein SEC6 or SEC15 was examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) was depicted to indicate that similar levels of RAB27b protein was present in each fraction.
(E) Endogenous RAB27b immunoprecipitated from uninfected or infected BECs. The association between RAB27b and endogenous Exocyst complex proteins SEC6 or SEC15 in uninfected or infected BECs were examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) were also depicted to indicate that similar levels of RAB27b, SEC6 or SEC15 protein were present in each fraction.
(F) Endogenous MyRIP immunoprecipitated from uninfected or infected BECs. The associations between MyRIP and endogenous Exocyst complex protein SEC6 or SEC15 in naïve or infected BECs were examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) were also depicted to indicate that similar levels of MyRIP, SEC6 or SEC15 protein were present in each fraction.
(G) Protein immunoblot analysis of RAB11a in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from infected BECs which are pre-transfected with control shRNA or shRNA targeting Exocyst complex protein SEC6 or SEC15.
(H) Protein immunoblot analysis of MyRIP or MyosinVIIa in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from infected BECs which are pre-transfected with control shRNA or shRNA targeting Exocyst complex protein SEC6.
(I) Rab11-binding domain (R11BD) from the RAB11a effector RAB11FIP3 and the Rab27b-binding domain (R27BD) from the RAB27b effector SLAC2 were cloned and fused to GST. The purified R11BD-GST or R27BD-GST proteins were immobilized and used to pulled down GTP-bound active RAB11a or RAB27b from uninfected or infected BECs transfected with control shRNA or shRNA targeting SEC6 or SEC15. The proteins of interest in the total cell lysates (TCL) were depicted to demonstrate that similar levels of RAB11a or RAB27b protein were present in each fraction.
(J) Immunoprecipitation of RAB11a from uninfected or infected BECs transfected with control shRNA or shRNA targeting SEC6 or SEC15. The association between RAB11a with its effector RAB11FIP3 in control or KD cells were examined by western blotting of the IP fractions. The proteins of interests in the total cell lysates (TCL) were depicted to demonstrate that similar levels of RAB11FIP3 protein was present in each fraction.
(K) Immunoprecipitation of RAB27b from uninfected or infected BECs transfected with control shRNA or shRNA targeting SEC6 or SEC15. The association between RAB27b with its effector MyRIP in control or KD cells were examined by western blotting of the IP fractions. The proteins of interests in the total cell lysates (TCL) were depicted to demonstrate that similar levels of MyRIP protein were present in each fraction. See Figure S3 and S4
