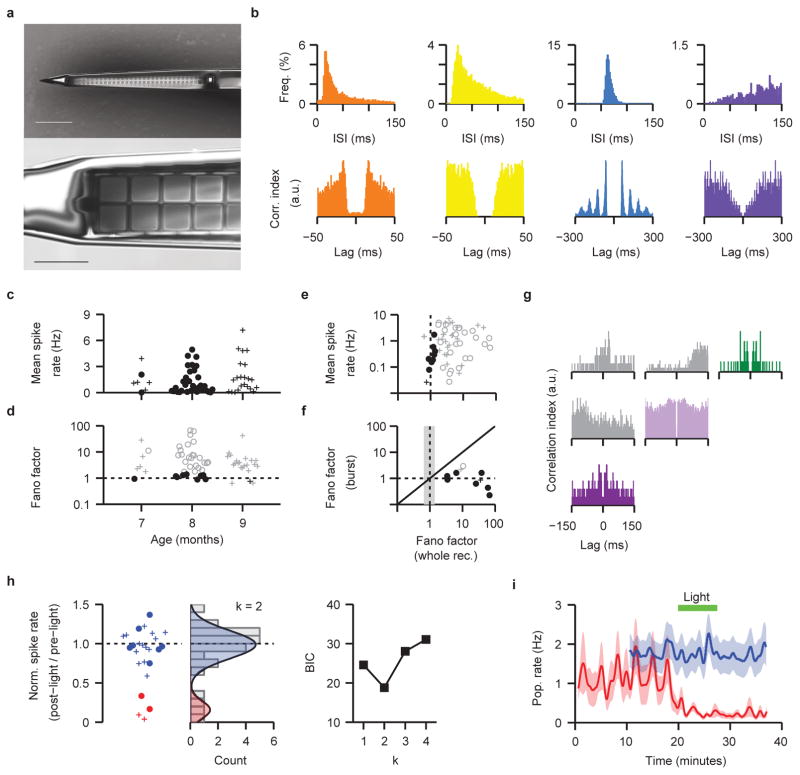
Extended Data Figure 7. Statistical structure of spontaneous firing activity in human brain organoids.
a. Electron micrograph of a 64-channel high-density probe shank (corresponding to the tip of the device shown in Fig. 4a) with two columns of 32 rows of recording sites, visible as squares along the center of the shank (top panel). The recording sites consist of 9×9 μm exposed gold pads (bottom panel) that are PEDOT electroplated to lower electrical impedances. Each recording site is connected to the outside by insulated nanofabricated wires, running along the length of the probe shank (visible as gray lines flanking the recording sites). The shank width is tapered from 35 to 60 μm wide, and is 15 μm thick. Scale bars, 100 μm (top), 20 μm (bottom). b. Example inter-spike interval (top) and auto-correlogram (bottom) plots for spontaneous activity recorded from 4 prototypical units; 1 ms bins. c. Plot of the mean spike rate in iPSC11a (o) and HuES66 (+) organoids recorded at 7–9 months. The difference between the median of the mean spike rates in the two organoid lines are non-significant (iPSC11a n= 34 cells, 12 recording sites, 7 organoids, M= 0.662, Q1= 0.186, Q3= 2.071; HuES66 n= 27 cells, 9 recording sites, 8 organoids, M=1.186, Q1= 0.463, Q3= 2.848; 2 tailed Wilcoxon rank sum test, 5% significance level, z= -1.47, p= 0.14; squared rank test suggests that the variance of mean spike rate in the two organoid lines is not significantly different, z= -1.47, p= 0.141). d. Plot of the fano factor against organoid age. e. Plot of mean spike rate against the fano factor (n = 61 neuronal units, from 15 organoids). Fano factors that are outside the expected 99% confidence bounds are plotted in gray; those that are within the 99% confidence bounds are plotted in black. A time-series fano factor greater than 1 indicates that the unit firing is not well modeled by a stationary Poisson distribution, and points at the presence of network activity. f. Plot of the fano factor calculated across the whole recording versus the fano factor calculated during the population bursts. Shaded region highlights the 99% confidence bounds for a whole-recording fano factor of 1. Black symbols correspond to units with a fano factor close to 1, consistent with a stationary Poisson distributed system and implying a fixed firing profile during the first second of population burst. g. Spike train auto-correlogram (color) and cross-correlogram (gray) for the three units presented in Figure 4g. Color-coding as in Figure 4g. h. Left, baseline normalized spike rate (n = 25 cells from 10 organoids; o, iPSC11a, n = 9 cells 4 organoids; +, HuES66, n = 16 cells 6 organoids); color identifies the clustering of data points into responders (red) and non-responders (blue). Middle, count histogram of the data plot in the left panel overlaid by the optimal Gaussian mixture model (number of components k = 2; see Methods). The two underlying distributions are plotted as shaded regions (μ1= 0.16, σ1= 0.11, mixing proportion= 0.16; μ2= 0.99, σ2= 0.18, mixing proportion= 0.84). Right, plot of the Bayes Information Criterion (BIC) generated for the Gaussian mixture models with k components. BIC is minimized at k = 2, signifying that the data is best described by a bimodal distribution (see middle panel). i. Population averaged activity for the two populations of neurons (responders in red, n=4 neurons; non-responders in blue, n=21 neurons); error envelope (shaded) is the s.e.m. In the 4 responsive organoids, light stimulation attenuated firing rate in 4 out of 5 isolated neurons.