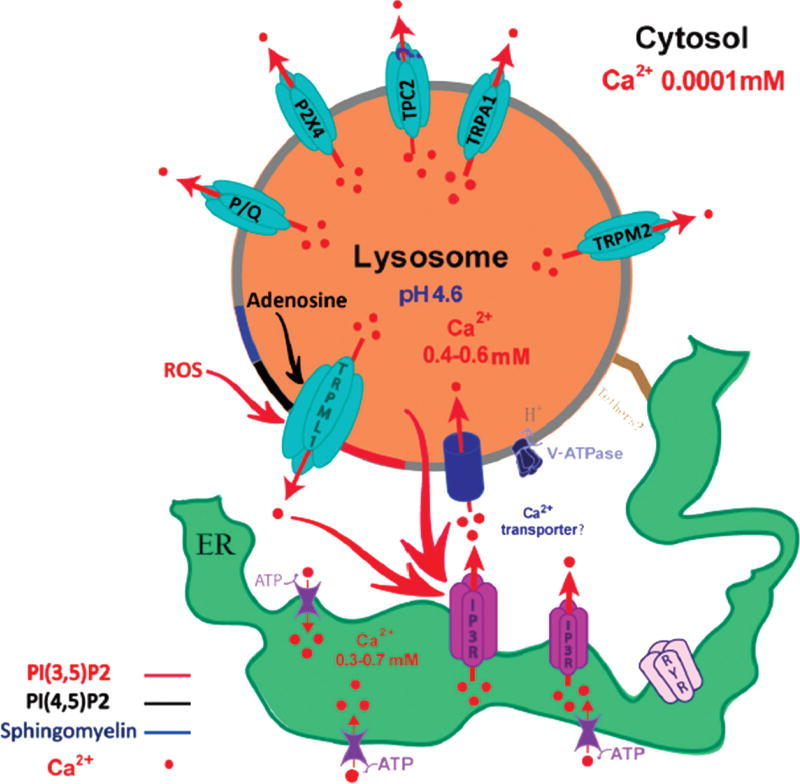
Figure 1.
Lysosomal Ca2+ release channels and store refiling mechanisms. Several Ca2+-permeable channels may be present on the lysosomal membranes. Activation of the channels trigger Ca2+ release from lysosomal Ca2+ stores. Among them, the gating mechanisms are most well-established for TRPML1. The lysosome-enriched phosphoinositide PI(3,5)P2 (red) can activate the channel, while plasma-membrane-enriched phosphoinositide PI(4,5)P2 (black) can inhibit TRPML1. Additionally, TRPML1 can be activated (red arrow) by reactive oxygen species (ROS), and ROS-induced lysosomal Ca2+ release may induce lysosomal biogenesis (Zhang et al., 2016). In addition, accumulated materials such as sphingomyelin (blue) and adenosine may inhibit (black arrow) TRPML1. While TPC-mediated lysosomal Ca2+ release can be triggered by NAADP or sphingosine (Calcraft et al., 2009; Zong et al., 2009; Lloyd-Evans et al., 2010; Hoglinger et al., 2015) (whether TPC2 serves as the Ca2+ channel still remains convtroversial, indicated by the question mark), activation mechanisms for other lysosomal Ca2+ channels are less clear. Upon lysosomal Ca2+ channel activation, lysosome stores are largely depleted, which is expected to trigger a refilling mechanism. In refilling mechanism 1, a putative Ca2+–H+ exchanger may transport Ca2+ ions into lysosome lumen in an H+-dependent manner (Morgan et al., 2011). Alternatively, Garrity et al. suggest that in the ER-lysosome membrane contact sites, IP3Rs–mediated ER release Ca2+ may allow an unidentified Ca2+ transporter to transport Ca2+ to lysosomes. In this model, possible tether proteins may link ER membrane proteins directly with lysosomal membrane proteins at ER-lysosome membrane contact sites. Reprinted with permission from Garrity, A. G., et al. (2016). The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. Elife 5, e15887. © 2016, eLife/CC BY 4.0.