Figure 41.
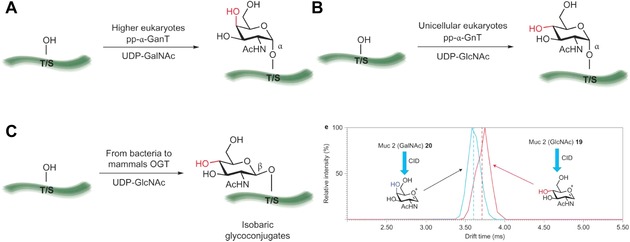
Examples of common epimeric glycoconjugates and families of enzymes involved in their biosynthesis. (a) The pp‐α‐GnT family of enzymes mediate the transfer of an α ‐linked GalNAc residue to the hydroxyl groups of serine and threonine residues in proteins. This modification is common in higher eukaryotes (including humans) and represents the first step in the biosynthesis of mucin‐type O‐glycans. (b) In unicellular eukaryotes, mucin‐type O‐glycans have been found initiated with an α‐linked GlcNAc residue generated by a pp‐a‐GnT family of enzymes. (c) β‐linked GlcNAc is a common post‐translational modification (PTM) observed in many organisms and is especially prevalent among multicellular eukaryotes. The attachment of this residue is mediated by O‐GlcNAc transferase (OGT) β‐linked GlcNAc is also transferred to the notch epidermal growth factor repeats by extracellular OGT. The differentiation of epimeric glycopeptides by mass spectrometry has not been reported previously, (d) Travelling wave ion mobility spectrometry mass spectrometry (TWIMS) arrival time distribution showing the discrimination of epimeric glycopeptides 19 and 20 and the distinction of HexNAc oxonium ions generated following collision induced dissociation (CID). CID of glycopeptides 19 and 20 before ion mobility separation results in the formation of epimeric oxonium ions that are distinguishable by TWIMS‐MS. Vertical dashed lines represent drift time as identified by full width half height. Reprinted by permission from Macmillan Publishers Ltd: Nature Chemistry,525 copyright 2014.
