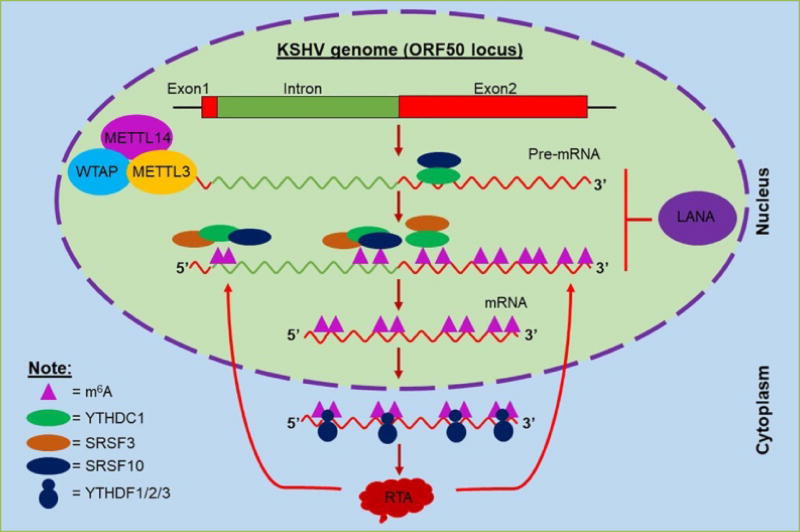
Figure 1. Post-transcriptional m6A modification controls KSHV RTA (ORF50) pre-mRNA splicing.
Multiple m6A sites are found in RTA pre-mRNA, which are methylated by m6A writers METTL3, METTL14, and WTAP. The m6A sites in the intron near the two splicing sites are critical for YTHDC1 binding and recruitment of splicing factors SRSF3 and SRSF10 while the m6A site in Exon2 near the splicing site is important for recruitment of SRSF3 and dissociation of SRSF10. Interactions between the m6A-modified RTA pre-mRNA and the different splicing factors ensure exclusion splicing of the intron to generate RTA mRNA. The other m6A sites may enhance RTA mRNA export, stability, and translation through interaction with m6A readers YTHDF1, YTHDF2, and YTHDF3. The expressed RTA protein enhances the host’s m6A machinery to increase the levels of m6A to promote its own pre-mRNA splicing and KSHV lytic gene expression. In contrast, KSHV latent protein LANA has the opposite effects on m6A and RTA pre-mRNA splicing.