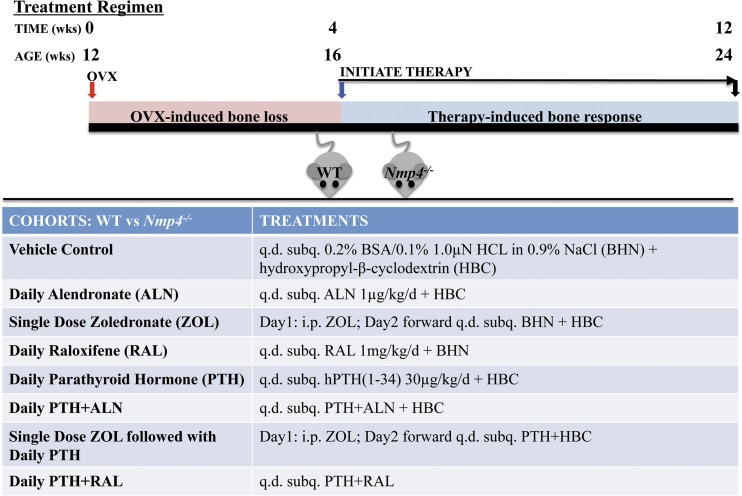
Figure 1.
WT and Nmp4−/− mice were ovariectomized (ovx’d) at 12 weeks of age. At 16 weeks of age, the mice were sorted into 16 treatment groups by weight and genotype. Each mouse received two sequential 100-µL injections per day (q.d.) containing the drugs or vehicle(s), as shown for 8 weeks. Mice were euthanized, and the bones were processed for analysis at 24 weeks of age. WT and Nmp4−/− mice were administered the following treatments: (1) Vehicle-control: inject subcutaneously (subq.) 100 µL 0.2% bovine serum albumin/0.1% 1.0 µN HCl in 0.9% NaCl (BHN diluent for PTH/ALN) + 100 µL 20% hydroxypropyl-β-cyclodextrin (HBC diluent for RAL diluent). (2) Daily ALN: inject subq. 100 µL ALN at 1 μg/kg/d + 100 µL HBC. (3) ZOL: on day 1 of treatment, inject intraperitoneally (i.p.) 100 µL ZOL at 80 μg/kg in phosphate-buffered saline. On day 2 forward, inject subq. 100 µL BHN + 100 µL HBC. (4) Daily RAL: inject subq. 100 µL RAL at 1 mg/kg/d + 100 µL BHN. (5) Daily PTH: inject subq. 100 µL synthetic human PTH 1–34 acetate salt (Bachem Americas, Inc.), at 30 µg/kg/d + 100 µL HBC. (6) Daily PTH + ALN: inject subq. 100 µL PTH/ALN + 100 µL HBC. (7) Single-dose ZOL followed by daily PTH: on day 1 of treatment inject i.p. 100 µL ZOL. On day 2 forward, inject subq. 100 µL PTH + 100 µL HBC. (8) Daily PTH + RAL: inject subq. 100 µL RAL + 100 µL PTH.