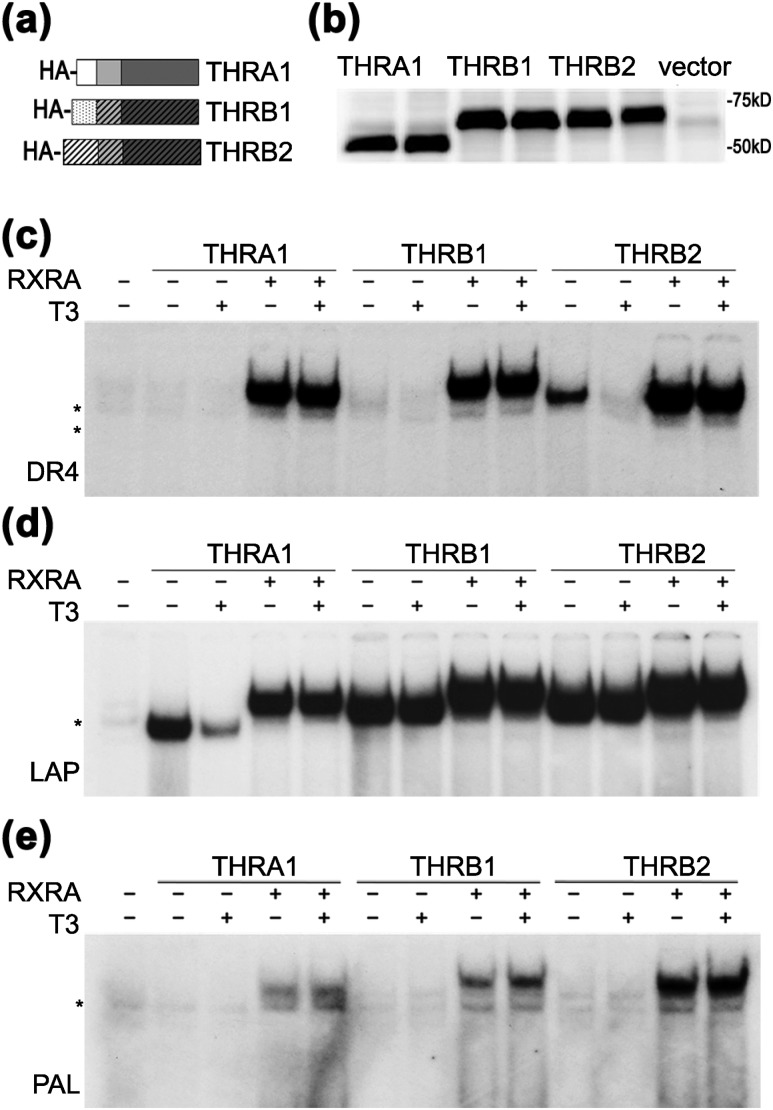
Figure 1.
THR isoforms have different abilities to form homodimers on DNA. (a) Schematic representation of hemagglutinin (HA)-tagged THR isoforms. AF-1, DBD, and LBD domains (N to C termini) are shown by different shading. (b) In vitro transcription/translation proteins from rabbit reticulocyte lysate were analyzed in duplicate by western blot using an anti-HA antibody. pSP72 was used as an “empty” vector negative control. (c–e) Binding of wild-type THRs to DR4, LAP, and PAL in the presence or absence of retinoic X receptor α (RXRA) were tested by electrophoretic mobility shift assay. (c) On a DR4 element, THRA1 bound exclusively as a heterodimer, THRB1 showed weak homodimer formation, and THRB2 bound as homodimer in the absence of T3. All THR isoforms heterodimerized with RXRA. (d) On an LAP element, all THRs isoforms bound as homodimers and heterodimers, but only THRA1 binding was dissociated by T3. (e) On a PAL element, THRs bound exclusively in the presence of RXRA. Samples were treated with vehicle or 100 nM T3. *Nonspecific band observed in unprogrammed lysate.