Figure 1.
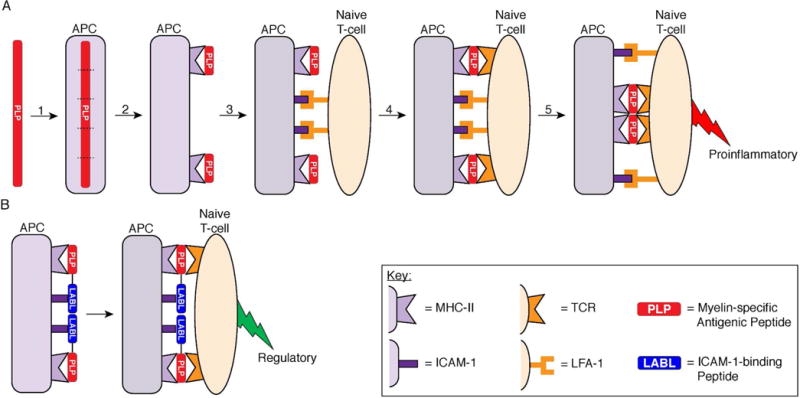
Two-signal model of T-cell activation highlighting the role of the ICAM-1–LFA-1 receptor pairing for both adhesion and costimulation and the alteration of T-cell activation by a BPI. (A) Mechanism of proinflammatory T-cell activation against myelin protein. (1) Myelin-specific antigen is internalized by an APC and broken into fragments. (2) The antigen fragments are presented on the APC cell surface by MHC-II. (3) A naïve CD4+ T-cell binds to the APC through the adhesion receptor pairings of ICAM-1 on the APC and LFA-1 on the T-cell. (4) If the naïve T-cell contains TCRs capable of recognizing the particular antigen presented by MHC-II, then the TCR binds the Ag-MHC-II complex to initiate signal-1 on the periphery. The ICAM-1–LFA-1 receptor pairings also function as costimulatory molecules to constitute signal-2 in a central cluster. (5) The two signals reorganize so that signal-1 pairings localize in the central cluster and the signal-2 pairings localize to the periphery of the signal-1 cluster to form the immunological synapse, which directs the maturation of a naïve T-cell into a proinflammatory T-cell against the myelin antigen. (B) Intervention of myelin-specific T-cell activation by a BPI. A BPI molecule consists of two peptides, a myelin-specific antigenic peptide (PLP) and a signal-2 blocking peptide (LABL), conjugated together. The antigenic peptide of the BPI allows formation of Ag–MHC-II pairing with the TCR to form signal-1 but inhibits the ICAM-1–LFA-1 receptor pairing to prevent formation of signal-2. Because the BPI peptides are conjugated together, the BPI is hypothesized to inhibit formation of the IS and alter the maturation of the naïve T-cell into a regulatory T-cell.
