Figure 2.
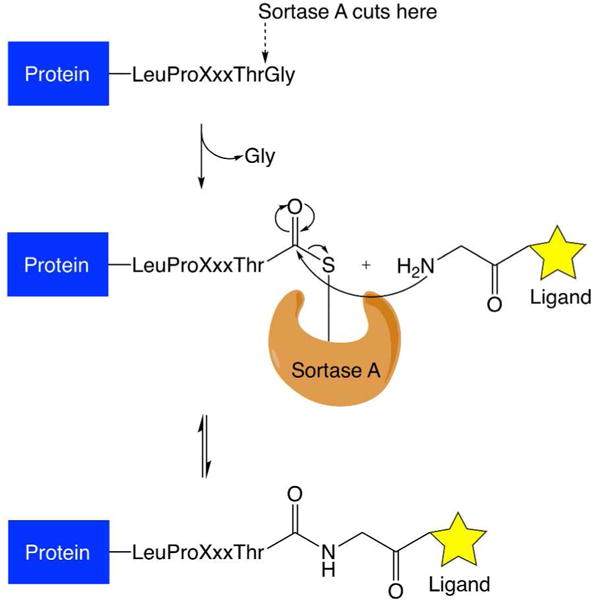
Mechanism of sortase-mediated ligations. The ligation requires a substrate to bear a LPXTG peptide tag. This peptide tag is recognized by sortase A. The enzyme cleaves between threonine and glycine residues to form an acylenzyme intermediate. The acyl-enzyme intermediate is then susceptible to nucleophilic attack by a ligand bearing an N-terminal glycine residue, leading to the formation of an amide bond between the substrate and the ligand.
