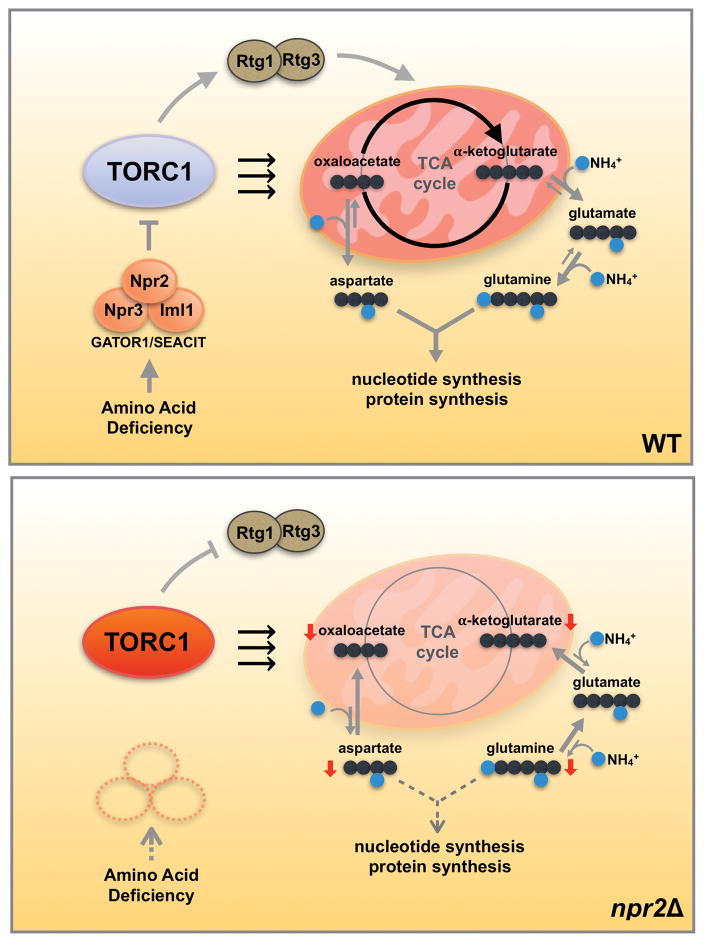
Figure 5. The GATOR1/SEACIT complex regulates cataplerotic reactions of the mitochondrial TCA cycle in tune with the amino acid and nitrogen status of cells.
In glucose medium, yeast cells preferentially perform glycolysis and reduce mitochondrial TCA cycle activity. When starved for amino acids (SD medium), WT cells are able to sense the lack of amino acids and regulate TORC1 activity through Npr2p and the GATOR1/SEACIT complex. As a result, WT cells boost TCA cycle activity as well as the retrograde response pathway for the production of nitrogen-containing glutamine and aspartate from α-ketoglutarate and oxaloacetate (cataplerotic reactions). Glutamine and aspartate are then consumed for nucleotide and protein synthesis, which have a high nitrogen demand. In npr2Δ mutants, cells inappropriately perceive themselves to be amino acid-replete. Constitutively active TORC1 inhibits the replenishment of α-ketoglutarate and oxaloacetate (anaplerotic reactions) due to dysregulation of the retrograde response pathway, and thus utilization of the mitochondria for synthesis of aspartate and glutamine is reduced due to the perception that cells are already amino acid-replete. Therefore, the GATOR1/SEACIT complex regulates TORC1 to balance cataplerotic and anaplerotic reactions of the TCA cycle in tune with the amino acid and nitrogen status of cells. Since glutamine and particularly aspartate rescue the consequences of loss of GATOR1/SEACIT, high TORC1 activity signifies that cells have the appropriate capacity for biosynthetic demands requiring these amino acids.