Figure 1. Stress-Activated Chaperones Ensure Bacterial Survival.
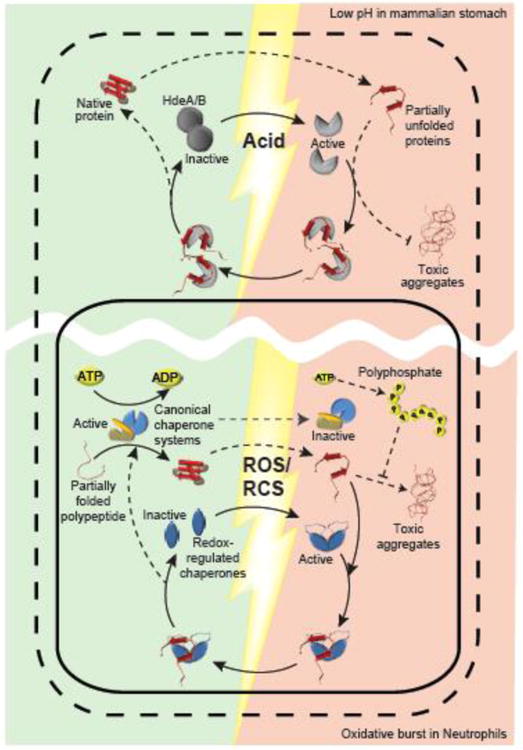
Bacteria are exposed to extremely fast acting stress conditions that have evolved as part of the mammalian host defense. Ingested gram-negative bacteria that colonize the intestine need to survive the highly acidic environment of the stomach. Their porous outer membrane (dashed line) allows the free passage of small molecules such as protons causing the sudden acid-induced unfolding of periplasmic proteins (red shaded area). To deal with these stress conditions, Gram-negative bacteria employ the periplasmic chaperones HdeA and HdeB (grey), which undergo rapid acid-induced unfolding and dissociation, activating their ATP-independent chaperone function. Chaperone-active HdeA/B bind unfolding proteins (red), maintain their solubility and prevent the accumulation of toxic protein aggregates. Upon return to neutral pH (green shaded area), HdeA mediates client protein refolding concomitant with its own refolding. Another mammalian host defense involves the production of high levels of reactive oxygen or chlorine species (ROS/RCS), such as H2O2 and HOCl, respectively at gut epithelia and in macrophages. ROS/RCS-mediated side chain modifications cause protein unfolding and aggregation in the cytosol (red shaded area within solid line). In addition, ROS/RCS cause a decline in cellular ATP levels, and directly inhibit canonical ATP-dependent chaperones. In bacteria, ATP is largely rerouted to polyphosphate, which functions as chaperone itself. To cope with this stress, bacteria employ redox-regulated ATP-independent chaperone such as Hsp33 and RidA (blue), which sense ROS/RCS through disulfide bond formation or N-chlorination of amino acid side chains, respectively. Chaperone-active Hsp33/RidA bind unfolding proteins, prevent their aggregation and maintain their solubility. Once reducing, non-stress conditions and ATP levels are restored, canonical chaperones release and refold the unfolded client proteins bound to Hsp33. So far it remains unclear whether RidA can transfer its substrate to canonical chaperones for refolding.
