Figure 3. Peroxiredoxins - A Multi-stress Sensing Dual-Function Protein Family.
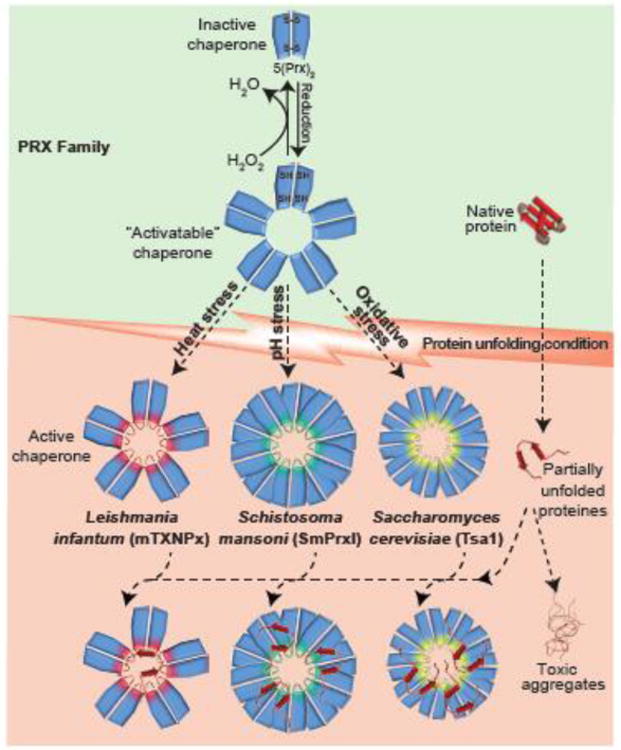
Members of the peroxiredoxin (PRX) family (blue) effectively detoxify peroxide and other reactive oxygen and nitrogen species (green shaded area). As part of the oxidation/reduction process, PRX cycles between reduced decamers and oxidized dimers. Members of the PRX family have been found to switch into potent ATP-independent chaperones once specific protein-unfolding stress conditions are encountered. Leishmania infantum peroxiredoxin mTXNPx, for instance, specifically senses heat stress, whereas Schistosoma mansoni SmPrxI's responds to pH stress and Tsa1 from Saccharomyces cerevisiae is activated by high levels of ROS. The mechanism of stress sensing depends on the respective stress conditions (please see text for details) but the structural changes that the PRX members undergo appear to be similar. They include the formation of chaperone-active decamers and higher oligomeric states, which show increased exposure of hydrophobic surfaces, and decreased peroxiase activity. The chaperone-active peroxiredoxins bind unfolding proteins and hence prevent the formation of cytotoxic protein aggregates. While the binding of unfolding client proteins in the center of the ring-like structure has been shown for mTXNPx, it is unclear where the other PRXs bind their client proteins. mTXNPx interacts with canonical chaperone systems to release the client proteins and support their refolding. Stress-specific peroxiredoxins in parasites appear to be necessary for parasitic organisms to successfully propagate in the mammalian host.
