Figure 1. Structure of α-actinin dimmers.
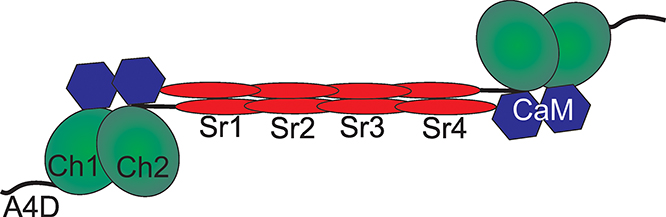
The active homodimer form of α-actinin consists of a pair of subunits in an anti-parallel arrangement. Three main functional domains exist in the monomer. At the N-terminus, two CH domains (CH1 and CH2) comprise an actin binding domain (ABD). A series of four spectrin repeats (SR1-4) form the long rod-shaped domain and is the main region of monomer association. At the C-terminus, a pair of EF domains comprise the calmodulinlike domain (CaM). A4D denotes 19 amino acid region of the N-terminus that is specific to the α- actinin-4 protein.
