Figure 2. Modes of α-actinin-4 regulation.
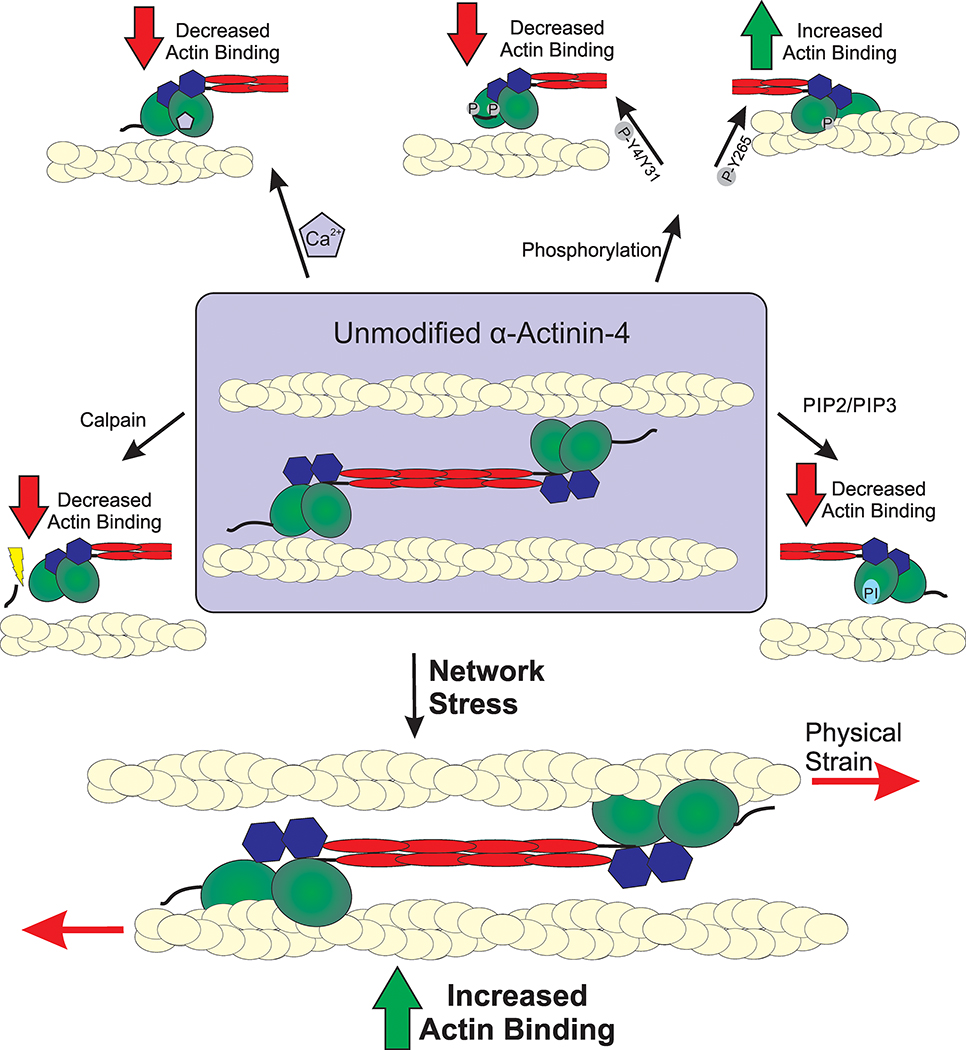
Actin binding activity of α-actinin-4 is mediated through a variety of regulatory mechanisms. The full dumb-bell shape dimer is displayed in the center with branching modes of regulation, showing only one half of a full dimer. Actin filaments are depicted as yellow cables. Calpain cleavage removes a regulatory N-terminal fragment that results in the reduction of actin-binding ability. Phosphorylation of α-actinin-4 results in both increased and decreased actin binding. Phosphorylation of Y4/Y31 residues closes the ABD and lowers actin affinity. A secondary phosphorylation site at Y265 disrupts the interaction between the CH domains and locks the ABD in a high affinity open state. Actin binding is decreased through binding of PIP2/PIP3 binding of the CH domains as well as Ca2+ binding of the C-terminal CaM domain, which lock the ABD in a closed state. The fifth regulatory mechanism of α-actinin-4 is sensation of mechanical stress on the actin cytoskeleton, which results in rapid accumulation to sites of external stress.
