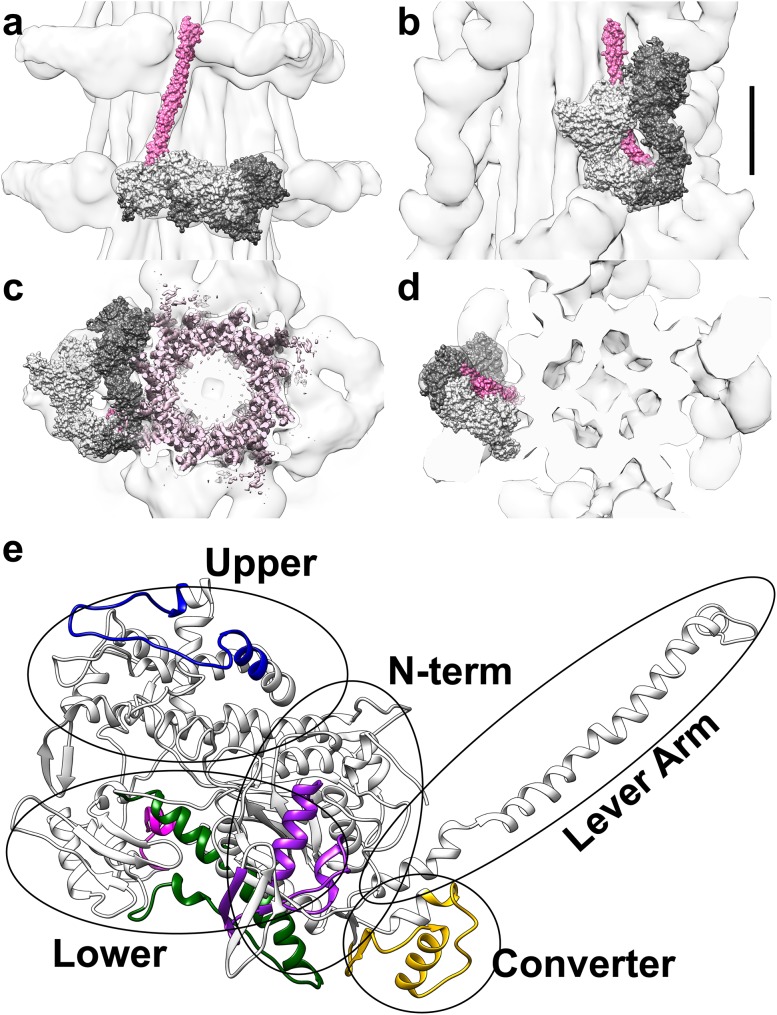
Fig. 1.
a–d Thick filament reconstructions from Lethocerus (Hu et al. 2016) on the left (a, c) and tarantula (Alamo et al. 2016) on the right (b, d) are shown in longitudinal (a, b) and cross-section views (c, d). A space-filling model of the myosin IHM, PDB 3JBH (Alamo et al. 2016), is fit within both maps. Although myosin is a dimer, the two heads of the IHM are not equivalent. One head (light gray) is called blocked, because its actin-binding domain contacts the back of its partner, called the free head (dark gray). In b and c, the IHMs are oriented similarly, although the filaments are perpendicular in these two views. Thus, the IHM is perpendicular to the S2 domain (pink) in Lethocerus (a); whereas it folds back to lie on top of the S2 domain in tarantula (b). The thick filament bare zone is towards the top of the page (a, b) or below the plane of the page (c, d). Scale bar 100 Å. e Ribbon diagram of myosin S1 head from the tarantula model, residues 1-838 of PDB 3JBH.g (Alamo et al. 2016), shows the five regions expected to be alternatively spliced in Lethocerus, color coded purple, blue, dark green, magenta, and yellow for MXEs 1, 5, 7, 8 and 10, respectively. A sixth alternatively spliced region is expected within the helical rod domain (not shown), near the junction between S2 and light meromyosin (LMM). The N-terminal, upper and lower 50 kD, converter, and lever arm domains of the S1 head are circled and labeled