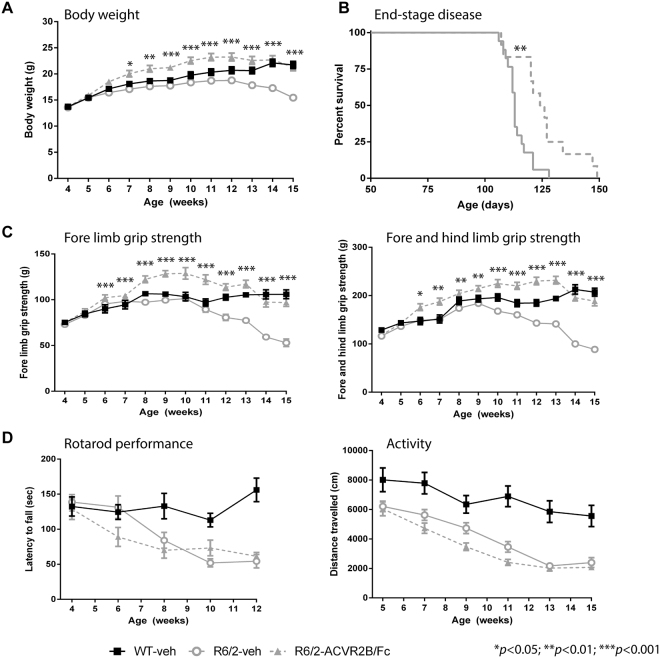
Figure 4.
Treatment with ACVR2B/Fc delays end-stage disease, but has no effect on rotarod performance or activity measures. (A) ACVR2B/Fc treatment completely prevented the progressive loss in body weight loss that occurs in R6/2 mice. The effect was such that R6/2 mice were significantly heavier than WT mice at some ages (Table S9). (B) Kaplan-Meier curve showing that ACVR2B/Fc treatment delays end-stage disease in R6/2 mice (Chi = 8.764, p < 0.01 Mantel-Cox log-rank test). (C) ACVR2B/Fc treatment completely prevented the progressive loss in fore-limb as well as the combined fore- and hind-limb grip strength that occurs in R6/2 mice. The effect was such that R6/2 mice were significantly stronger than WT mice at some ages (Table S9) (D) ACVR2B/Fc had no effect on the impairment in R6/2 rotarod performance or hypoactivity. Statistical analysis was two-way ANOVA with post-hoc Bonferroni correction (see Table S8 for main effects and Table S9 for multiple comparisons). The statistical significance between values for ACVR2B/Fc treated and vehicle treated R6/2 mice is depicted: *p < 0.05; **p < 0.01; ***p < 0.001. WT vehicle, n = 13; R6/2 vehicle, n = 17; R6/2 ACVR2B/Fc, n = 14. All data presented as means ± SEM.