Figure 4.
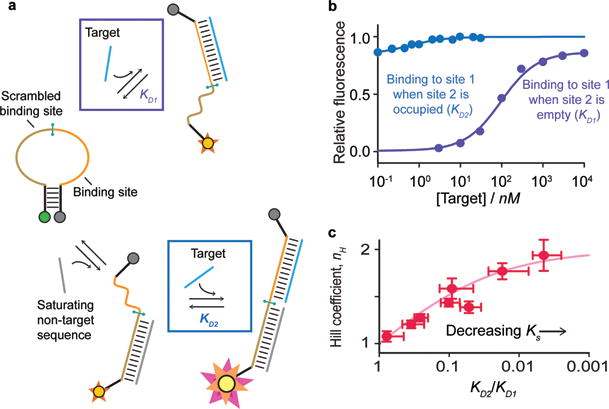
(a) The modular structure of our symmetric cooperative beacon allows exploration of the thermodynamics underlying its design. To do so we employed a construct in which the two ligand binding sites are distinct, allowing independent measurement of KD1 and KD2. (b) Shown are the affinities (at 39°C) measured when the other binding site is empty (purple), and when it is occupied (blue); note that, the latter occurs on a beacon that is already largely open, and thus produces only a fifth of the total signal change. The affinities obtained from this control predict nH = 1.88 for our sensor (eq. 3), within experimental error of the observed value (Figure 3). (c) Measuring KD2, KD1 (using the control construct), and nH (using the cooperative beacon) over a range of temperatures we find that the expected relationship between these values (eq. 3) holds even as the ratio of the binding affinities varies over orders of magnitude.
