Abstract
Alcohol use is often reported among people living with HIV/AIDS (PLWHA) and is associated with increased sexual risk and poor medication adherence. This meta-analysis evaluated the efficacy of behavioral interventions addressing alcohol use among PLWHA. Twenty-one studies (N = 8,461 PLWHA) that evaluated an individual-level intervention addressing alcohol use alone or as part of a more comprehensive alcohol/HIV intervention, included a control condition, and were available through December 2016 were included. Independent raters coded study, sample, and intervention content. Weighted mean effect sizes, using random-effects models, were calculated. Results indicate that interventions reduced alcohol consumption, increased condom use, and improved medication adherence relative to controls (d+s = 0.10–0.24). Plasma viral load was also reduced in intervention versus control participants (d+ = 0.14, 95% CI = 0.02, 0.26; k= 7). These findings show that behavioral interventions addressing alcohol use can successfully reduce alcohol consumption and also improve HIV-related outcomes among PLWHA.
Keywords: people living with HIV, alcohol, intervention, meta-analysis
INTRODUCTION
Globally, more than 36-million people currently live with HIV/AIDS, with sub-Saharan Africa bearing the heaviest burden, accounting for nearly 70% of all people living with HIV/AIDS (PLWHA) (1). Alcohol consumption is common among PLWHA with more than one-third reporting recent alcohol use across multiple geographical regions (2). In a U.S. sample of PLWHA linked to care, 8% were heavy drinkers (defined as drinking ≥5 drinks on a single day for ≥5 or more days in the past 30 days); among those who reported drinking in the past month, 15% were heavy drinkers – more than double the prevalence of heavy drinking in the general population (3, 4). A meta-analysis of studies conducted in Africa that examined the alcohol-HIV association (5) found drinker status to be associated with HIV infection such that drinkers were 70% more likely to be HIV-infected than non-drinkers and the risk of HIV was notably higher among problem drinkers than among non-problem drinkers. Furthermore, harmful alcohol use by PLWHA is of concern given that alcohol use is associated with the transmission of HIV, poor HIV management, and poor HIV treatment outcomes (6). Therefore, reducing alcohol consumption among PLWHA can help to reduce the transmission of HIV and promote medical adherence and subsequent viral suppression.
Behavioral interventions for PLWHA have largely focused on reducing sexual risk behaviors or improving medication adherence. The limited attention to alcohol use interventions for PLWHA is a missed opportunity given the consistent findings that alcohol consumption, especially problematic alcohol use, weakens the immune system, thus worsening the disease course (7). In contrast, a small, but growing body of research evaluating interventions targeting alcohol use alone or in the context of a broader HIV intervention (e.g., reducing sexual risk behaviors by addressing situations in which alcohol is consumed) has emerged over the past decade. Prior narrative reviews of interventions focused on alcohol use among PLWHA have found mixed results likely due to the nature of the intervention (i.e., single vs. multiple health behavior change target) (6, 8, 9). Researchers have advocated for a multiple behavioral change approach when intervening on behaviors that are related to one another such as alcohol and risky sexual behavior but there is limited evidence showing that multiple health behavior change interventions are superior to interventions addressing a single health behavior (10, 11).
Therefore, the purposes of this systematic review and meta-analysis were (a) to evaluate the efficacy of behavioral interventions addressing alcohol use alone or as part of a more comprehensive alcohol/HIV intervention, (b) to determine whether single-focus (i.e., alcohol use alone) interventions or multiple-focus (i.e., multiple risk behaviors) interventions are more efficacious, and (c) to examine study, sample, and intervention characteristics as potential moderators of the observed intervention effect. We expected that intervention participants would report greater reductions in alcohol use, fewer sexual risk behaviors, and improved medication adherence relative to controls across all studies. We also expected that the magnitude of these effects to differ by intervention target. If the intervention targeted alcohol use alone, we expected the intervention would be successful in reducing alcohol use but the success of the intervention to reduce other behaviors (when assessed) would be weaker given that participants were not provided with any intervention content (e.g., education, goal setting/harm prevention planning, stress management) to assist with their sexual risk reduction or medication adherence behaviors. We expected that participants exposed to a broader alcohol/HIV intervention would reduce their alcohol use, reduce their sexual risk behaviors, and increase their medication adherence relative to controls.
This meta-analysis differs from prior reviews of the literature (6, 8, 9) in four ways. First, we included interventions that explicitly addressed (and measured) alcohol use (not broad substance use) alone or as part of a more comprehensive intervention to determine the efficacy of interventions that include an alcohol component. Second, we included studies with a control or comparison condition (i.e., no single-group pretest-posttest designs) to determine the impact of alcohol-related interventions relative to controls. Third, we evaluated the impact of single-vs. multiple-focus alcohol-related HIV interventions to establish the potential benefit of multiple risk behaviors vs. single-focused alcohol interventions. Finally, we evaluated moderators of intervention efficacy to isolate for whom, where, and how interventions succeed at improving alcohol use, sexual risk behaviors, and medication adherence among PLWHA.
METHODS
The conduct and reporting of the current meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12); the PRISMA checklist can be found in the Supplementary Materials (S1).
Eligibility Criteria
Studies were included if they: (a) sampled people living with HIV/AIDS, (b) examined an individual-level intervention addressing alcohol use alone or as part of a more comprehensive alcohol/HIV intervention, (c) used a randomized controlled trial (RCT) or a quasi-experimental design that included a control or comparison condition, (d) measured drinking outcomes (e.g., quantity of alcohol consumed per drinking day, heavy drinking), (e) provided data needed to calculate effect sizes, and (f) were available via print or electronic journals, interlibrary loan, or from the authors (including electronic publications and dissertations) and obtained by December 2016. Studies were excluded if the (a) studies evaluated a pharmacological, mass media, or structural-level alcohol/HIV intervention, (b) intervention did not address alcohol use, and (c) assessment plan did not include a measure of alcohol use at post-intervention.
Information Sources and Search Strategy
Studies were identified using: (a) electronic bibliographic databases, (b) database and document repository held by The Meta-Analyses on Alcohol Use, Sexual Risk Behaviors, and HIV (MASH) Team at The Miriam Hospital (PI: Lori A. J. Scott-Sheldon, PhD), which has accumulated a database of published and unpublished research on alcohol and sexual/HIV risk behavior, (c) reference sections of relevant papers, (d) scientific journals, and (e) databases of funded research (i.e., NIH RePORTER, ClinicalTrials.gov). First, we searched multiple electronic reference databases (PubMed, PsycINFO, ProQuest Dissertations and Theses Full Text, CINAHL, ERIC, Global Health, SocIndex, The Cochrane Library, and Web of Science [social sciences and science citation indices]) using a Boolean search strategy: (“people living with HIV/AIDS” OR “people living with HIV” OR “people living with AIDS” OR “HIV-positive” OR “HIV+” OR “HIV seropositive” OR “HIV-infected” OR “HIV patients”) AND ((bing* AND drinking) OR (binge AND drinkers) OR (heavy and drinking) OR (heavy and drinkers) OR “alcoholic beverages” OR “alcohol drinking” OR “alcohol abuse” OR alcoholic OR alcohol OR “alcohol-related disorders” OR alcoholism OR intoxicat* OR drunk* OR liquor) AND (intervention OR prevention). Our search statement was developed with the assistance of a medical sciences librarian in the Alpert Medical School of Brown University. Because many electronic databases have specific search methods (e.g., Medical Subject Heading [MeSH] terms used in PubMED are not available in other databases such as PsycINFO), our basic search strategy was modified based on the specific search parameters for each electronic bibliographic database. No language or other restrictions were applied. All electronic reference database searches were conducted in July 2016 and updated in December 2016.
Study Screening
All electronic bibliographic records (i.e., titles and abstracts) were initially screened for inclusion. Full-text manuscripts of potentially relevant records were retrieved and reviewed for final inclusion. Finally, reference sections of relevant manuscripts (including published reviews obtained through the electronic reference database searches) were also reviewed and included if they met the inclusion criteria. When authors reported details, ancillary information (e.g., results from the pilot study), and/or outcomes of a study in multiple manuscripts, the manuscripts were linked in the database and represented as a single unit (to avoid double-counting the same study). The manuscript reporting on the intervention outcomes was selected as the primary manuscript.
Data Collection and Reliability
Two independent coders extracted study information and setting (e.g., year, location), sample characteristics (e.g., gender, ethnicity), design and measurement specifics (e.g., recruitment strategy, method of assessment), risk characteristics (e.g., problem drinking) and intervention components (e.g., personalized alcohol feedback, condom communication skills-training). Methodological quality was assessed using 17 items (e.g., random assignment) from validated measures (13–15), with a maximum total quality score of 25. When multiple reports described the same study, relevant data were coded from both the primary and linked manuscripts. Inter-rater reliability was assessed for all study, sample, and methodological variables. There was a high degree of consistency. For the categorical variables, raters agreed on 88% of the judgments (mean Cohen’s κ = .71). Reliability for the continuous variables (e.g., proportion women) yielded an average intra-class correlation coefficient (ρ) of 0.85 across categories (median = 1.00). Disagreements between pairs of coders were resolved through discussion (e.g., coder identified manuscript page number where the information could be found) and the coding was revised. All revisions to the data were reviewed by the first author.
Study Outcomes
Intervention efficacy was determined from self-reports of alcohol use. Measures of alcohol use included (a) proportion of participants who consumed alcohol, (b) frequency of drinking days, (c) quantity of alcohol consumed, and (d) heavy episodic drinking (i.e., defined as 5 [4] or more drinks per occasion for men [women] over a period of time (30–90 days) (16–18). We also determined the efficacy of each intervention to reduce sexual risk behaviors (i.e., number of sexual partners, condom use, and sexual risk composite). The sexual risk behaviors of condom use (e.g., proportion or number of protected sex events, consistent condom use) and sexual risk composite (e.g., total number of sexual risk behaviors) were assessed using multiple methods and collapsed across measures to obtain single item of condom use and sexual risk. Finally, the efficacy of the interventions to improve adherence were determined by plasma viral load as well as self-reports of medication adherence.
Risk of Bias across Studies
We examined the studies for potential publication bias by (a) inspecting funnel plots (19) and (b) assessing the degree asymmetry in the distribution of effect sizes using Begg’s and Egger’s techniques (20, 21). Trim and fill procedures (22, 23) are used to estimate and correct for the possibility of missing studies (based on a rank-based data augmentation procedure) when publication bias is detected using the aforementioned funnel plot asymmetry tests (20, 21). Consistent with meta-analytic procedures, we conducted these tests only for dependent variables with 10 or more studies (24).
Summary Measures
Effect sizes (d) were calculated as the mean difference between the intervention and the control or comparison group (between-group) divided by the pre-test standard deviation (25, 26). The effect sizes were controlled for baseline when baseline statistics were provided (26). Other statistical information (e.g., t-tests) were used when means and standard deviations were not provided (27, 28). If a study reported dichotomous outcomes (e.g., frequencies), we calculated an odds ratio and transformed it to d using the Cox transformation (29). If the statistical information was unavailable (and could not be obtained from the authors) and the study reported a non-significant or significant difference, we estimated that effect size to be zero or, when a report noted the effect was significant, calculated an effect size based on the minimum statistically significant p-value (i.e., p = .05), respectively (28). All effect sizes were corrected for sample size bias (30); positive effect sizes indicated that participants who received the intervention reported reductions in their alcohol use or sexual risk behaviors and improvements in their medication adherence compared to controls. Two independent coders calculated effect sizes for each study, and discrepancies were resolved through discussion.
Synthesis of Results
Data analyses were conducted with Stata/SE 12.1 (31) using published macros (28, 32). Weighted mean effect sizes were calculated using random-effects procedures (28). The 95% confidence intervals (CIs) surrounding the weighted mean effect size were calculated; CIs indicate the degree of precision as well as the significance of the mean effect size (28). Heterogeneity in effect sizes was identified by computing Q and the associated degrees of freedom; a significant Q indicates a lack of homogeneity and an inference of heterogeneity. To assess the extent to which outcomes were consistent across studies, the I2 index and its corresponding 95% CIs were calculated (33, 34). The I2 values of 25%, 50%, and 75% are considered to be low, medium, and high heterogeneity (35).
Moderator tests were used to explain the variability in the effect size estimates. Moderator analyses were conducted using a modified weighted regression analysis or the meta-analytic analogue to the ANOVA (following random-effects assumptions) with weights equivalent to the inverse of the variance plus the random variance component for each effect size (28, 36). The proportion of between-study variation (T2) and residual variation due to heterogeneity (I2) were computed for the meta-regression analyses. The between-study heterogeneity (QB) for the meta-analytic analogue to the ANOVA was assessed. These analyses examined a priori determined moderators. That is, we expected that the study (i.e., geographical location, recruitment setting), sample (proportion women, proportion problematic alcohol use, and proportion currently on ART), or intervention length (total intervention dose) would be related to the variability in the between-group effect sizes.
RESULTS
Study Selection
Electronic database searches identified 4,060 records with relevant key terms (after removing duplicates). An additional 89 manuscripts were identified through other sources (e.g., reference sections of review papers). Of the 4,149 records reviewed, 3,176 records were excluded based on title and abstract review because those studies did not meet any of the inclusion criteria or were review papers. The full-text reports of the remaining 973 records were reviewed with an additional 856 records excluded because the study did not meet the inclusion criteria (e.g., no intervention, control group, or relevant outcomes). The final sample included 21 studies and 96 supplemental manuscripts that provided additional intervention details or data from the same sample reported in the primary paper (Figure 1) (16–18, 37–54).
Figure 1.
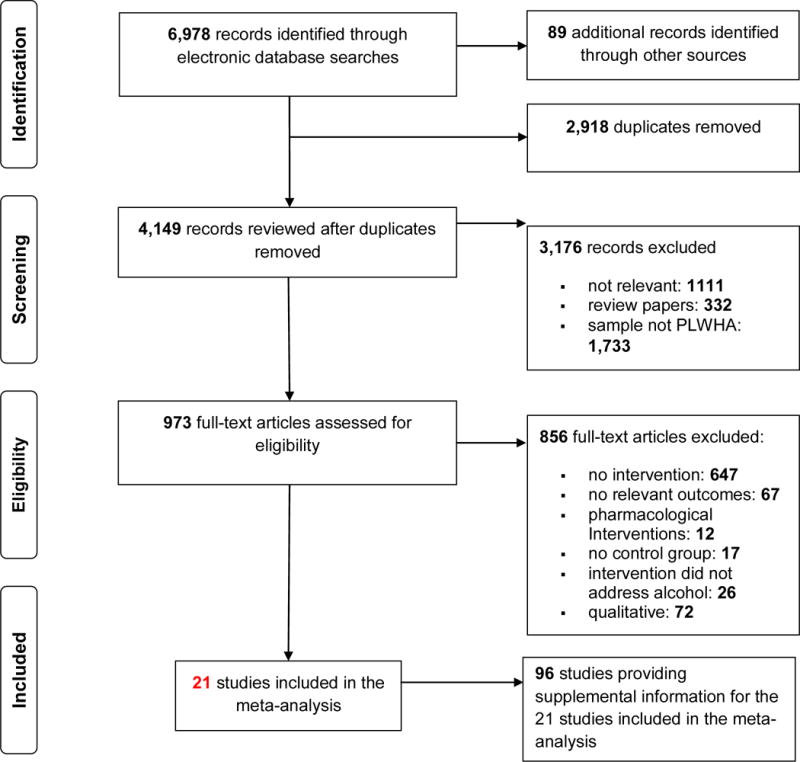
Screening and Selection Procedures
Study Characteristics
Table I provides study, sample, and intervention details for the 21 studies (k = 22 interventions) included in the systematic review and meta-analysis. Included studies were published (or available) between 2003 and 2016 (M = 2010, SD = 3.98); data collection occurred an average of 6 years earlier (M = 2004, SD = 4.85; range = 1994 to 2013). Studies were conducted in four World Health Organization defined geographical regions (55): 71% Americas (14 United States, 1 Haiti), 19% Africa (2 Uganda, 1 Kenya, and 1 multiple countries: Namibia, Kenya, and Tanzania), 5% South-East Asia (1 Thailand), and 5% European (1 Russia). Recruitment took place most often in a clinic (67%); samples were also recruited from the community (5%), prison (5%), or multiple clinic and community sites (24%).
Table I.
Study, sample, and intervention details for the 21 studies (22 interventions) included in the meta-analysis
| Citation | Sample | Setting and Location |
Baseline Alcohol |
Control | Intervention | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Theory/ Technique |
Target | Level | Session (no.) |
Dose (mins) |
||||||
| Bachanas et al. (37); Linked studies (63–68) | N = 3,538; 58% F; Mage = 37; 70% diagnosed with HIV ≤36 months; 64% ART | HIV clinics; Kenya; Namibia; Tanzania, Africa | 20% CD 5% PDa | WL/NT/AO | MF | Multiple (ALC; SEX; ADH) | IND/GRP | NR | NR | ALC; SEX; ADH |
| Chander et al. (16) | N = 148; 100% F; 86% Black; 70% ART | HIV clinic; Baltimore, MD, USA | 100% CD 100% PDb 55% AUDi | WL/NT/AO | NR | ALC | IND | 2 | 40 | ALC; SEX; PVL; ADH |
| Gilbert et al. (17); Linked studies (69, 70) | N = 471; 21% F; 50% Black; Mage = 44 | HIV clinics; San Francisco, CA, USA | 39% CD 39% PDc | WL/NT/AO | TTM; MI | Multiple (ALC; SEX) | IND | 1 | 24 | ALC; SEX |
| Hasin et al. (38); Linked studies (71–81) | N = 258; 22% F; 49% Black; Mage = 46; Mmonths living with HIV = 154; 77% ART | HIV clinic; New York, USA | 100% CD 100% PDd 48% AUD | INFO | Motivational Interviewing + HealthCall | ALC | ||||
| TTM; MI | ALC | IND | 3+ | 47.5 | ||||||
| Motivational Interviewing | ||||||||||
| Jean (39) | N = 116; 100% F; 95% Black; Mage = 36; 100% ART | Medical clinics; Port-au-Prince, Haiti | 100% CD 83% PDa | WL/NT/AO | IMB | Multiple (ALC; ADH) | GRP | 8 | 960 | ALC; ADH |
| Naar-King et al. (40); Linked studies (71, 82–88) | N = 186; 47% F; 83% Black; Mage = 21; 34% ART | Adolescent HIV clinics; Los Angeles, CA; Philadelphia, PA; Baltimore, MD; Fort Lauderdale, FL; Detroit, MI, USA | 53% CD 13% PDe | WL/NT/AO | TTM; MET | Multiple (ALC; SEX; ADH) | IND | 4 | 240 | ALC; PVL |
| Naar-King et al. (41); Linked studies (89–92) | N = 77; 48% F; 88% Black; Mage = 21 | Adolescent HIV clinic; USA | 77% CD | WL/NT/AO | TTM; MI | Multiple (ALC; SEX; ADH) | IND | 4 | 240 | ALC; SEX; PVL |
| Papas et al. (42); Linked studies (93–95) | N = 75; Mage = 37; 99% Black; Mmonths living with HIV = 16; 61% ART | HIV clinic; Eldoret, Kenya | 100% CD | WL/NT/AO | SCT | ALC | GRP | 6 | 540 | ALC |
| Parsons et al. (43); Linked studies (96–101) | N = 359; 21% F; 66% Black; Mage = 44; Mmonths living with HIV = 127; 100% ART | Behavioral research center; New York, NY, USA | 100% CD 100% PDf | INFO | IMB; MI | Multiple (ALC; ADH) | IND | 8 | 480 | ALC; ADH; CD4; PVL |
| Rongkavilit et al. (44); Linked studies (102) | N = 110; 19% F; 99% Asian; Mage = 22; 79% diagnosed with HIV in ≤24 months; 36% ART | AIDS research center clinics; Bangkok, Thailand | 36% CD | ICM | MI | Multiple (ALC; SEX; ADH) | IND | 4 | 240 | ALC; SEX; ADH; PVL |
| Rotheram-Borus et al. (45); Linked studies (96, 103–112) | N = 339; 100% F; 68% Latino/a; Mage = 40; Mmonths living with HIV = 109; 76% ART | Clinic and community sites; Los Angles, California, USA | 62% CD | WL/NT/AO | SCT | Multiple (ALC; SEX; ADH) | GRP | 16 | 1680 | ALC; SEX |
| Samet et al. (46); Linked studies (113–125) | N = 151; 19% F; 47% Black; Mage = 43; 100% ART | Clinics; Boston, MA, USA | 48% CD 100% PDg | WL/NT/AO | HBM; MET | Multiple (ALC; ADH) | IND | 4 | 570 | ALC; CD4; PVL; ADH |
| Samet et al. (47); Linked studies (126–138) | N = 700; 41% F; Mmonths living with HIV = 53; 24% ART | Infectious disease hospital; St. Petersburg, Russia | 100% CD 100% PDh 64% AUD | ICM | SCT; MET | Multiple (ALC; SEX) | IND/GRP | 5 | NR | ALC; SEX; STIs |
| Senyoni et al. (48); Linked studies (111, 139) | N = 186; 53% F; 100% Black; Mage = 15; Mmonths living with HIV = 180; 100% ART | HIV clinic, Kampala, Uganda | 3% CD | INFO | SCT | Multiple (ALC; SEX) | GRP | 8 | 640 | ALC; SEX |
| Sikkema et al. (49) | N = 65; 0% F; 51% White; Mage = 32; Mmonths living with HIV = 30 | HIV primary care clinic; New York, NY, USA | NR | INFO | IMB | Multiple (ALC; SEX) | IND | 3 | 180 | ALC; SEX |
| Sikkema et al. (50); Linked studies (140–148) | N = 247; 52% F; 68% Black; Mage = 42; Mmonths living with HIV = 120; 69% ART | AIDS services organization and community health care clinics; New York, NY, USA | 42% CD 13% PD 21% AUD | RCM | Stress and Coping Theory | Multiple (ALC; SEX) | GRP | 15 | 1350 | ALC; SEX |
| Sorensen et al. (51); Linked studies (149, 150) | N = 190; 27% F; 43% Black; Mage = 38 | San Francisco General Hospital; San Francisco, CA, USA | 61% CD | INFO | NR | Multiple (ALC; SEX; ADH) | IND | NR | NR | ALC; SEX |
| Velasquez et al. (18); Linked studies (63, 151–153) | N = 279; 0% F; 54% Black; Mage = 39; Mmonths living with HIV = 120 | Multiple community venues; New York, NY, USA | 100% CD 100% PDa 78% AUD | INFO | TTM; MI | Multiple (ALC; SEX) | IND/GRP | 8 | NR | ALC;SEX |
| Wandera et al. (52) | N = 337; 34% F; 32–46 years of age; 77% ART | Infectious disease clinic; Kampala, Uganda | 100% CD 69% PDa | RCNM | MI | ALC | IND | 1 | 25 | ALC |
| Weiss et al. (53); Linked studies (102, 154–156) | N = 482; 100% F; 42% Black; Mage = 42; 47% ART | Clinics and community sites; Miami, FL; New York, NY; NJ, USA | 11% CD 15% AUD | RCM | CBSM | Multiple (ALC; SEX; ADH) | IND/GRP | 16 | 2250 | ALC; PVL; ADH |
| Zack et al. (54); Linked studies (112, 157) | N = 147; 0% F; 55% Black; Mage = 38; Mmonths living with HIV = 60 | Prison; Marin County, CA, USA | 66% CD | WL/NT/AO | NR | Multiple (ALC; SEX) | GRP | 8 | 1080 | ALC; SEX |
Note. N, number of consenting participants; F, proportion female; CD, current drinker; PB, problematic drinking; WL/NT/AO, wait-list/no treatment/assessment only; INFO, informational/educational content only; ICM, irrelevant content, matched for time; RCM, relevant content, matched for time; RCNM, relevant content, not matched for time; MF, message framing (158); MI, Motivational Interviewing (159); TTM, Transtheoretical Model (160); Information-Motivation-Behavioral Skills (IMB) Model (161); SCT, Social Cognitive/Learning Theory (162); HBM, Health Belief Model (163); MET, Motivational Enhancement Theory (164); CBSM, Cognitive-Behavioral Stress Management (165); ALC, alcohol; SEX, sexual risk behaviors; ADH, adherence; IND, individual; GRP, group; CPLS, couples; PVL, plasma viral load; NA, not applicable; NR, not reported.
Problematic drinking defined as scoring 8 or more on the Alcohol Use Disorders Identification Test (AUDIT) (166).
Problematic drinking defined as 8 or more drinks per week OR 2 or more heavy drinking episodes (i.e., 4 or more drinks per drinking day) in the past 6 months.
Problematic drinking defined as ≥14 [7] drinks per week for men [women] per week OR 3 or more heavy drinking episodes (i.e., 5 [4] or more drinks per drinking day) in the past 3 months.
Problematic drinking defined as 4 or more drinks on at least one occasion in the past 30 days.
Problematic drinking defined as drinking 8 or more times in the past week.
Problematic drinking defined as ≥16 [12] drinks per week for men [women].
Problematic drinking defined as scoring 2 or more on the CAGE Questionnaire (167).
Problematic drinking defined as ≥14 [7] drinks per week for men [women] per week OR 3 or more heavy drinking episodes (i.e., 5 [4] or more drinks per drinking day) in the past 30 days.
Alcohol use disorders were clinically assessed in a subset (10%) of participants.
Sample Characteristics
In total, 8,461 PLWHA consented to participate in the studies (M = 403, SD = 736; range = 65 to 3,538); average retention was 82% (SD = 0.16). Samples included 43% women with a mean age of 36 (SD = 9.22; range = 15 to 46). Of the studies reporting race and/or ethnicity, 60% of the participants were Black (African or non-African), 19% were Asian, and 17% were White; 17% were Hispanic or Latino/a. Two studies exclusively sampled men who have sex with men (MSM).
Participants were diagnosed with HIV for an average of 8 years (M months = 97, SD = 54; range = 16 to 180; k = 10); 69% (SD = 0.25; range = 24% to 100%) reported currently taking antiretroviral medications. Baseline mean CD4 counts and plasma viral load was 417.61 (SD = 65.01; k = 8) and 3.17 (SD = 0.76; k = 4), respectively.
Most participants (M% = 66, SD = 0.33; range = 3% to 100%) reported recent alcohol use. Problematic drinking (see Table I for varying definitions) was assessed in 12 studies; in these studies, most participants (69%; SD = 0.40; range = 5% to 100%) were problem drinkers. When reported, alcohol use disorders were diagnosed in an average of 47% (SD = 0.25; range = 15% to 78%; k = 6) of the participants assessed. About half of the participants (51%; SD = 0.17; k = 11) reported using drugs other than alcohol. Of the five studies assessing intravenous drug use (IDU), an average of 30% of the participants (SD = 0.35) reported IDU. The use of alcohol or drugs concurrent with sex was reported by 48% (SD = 0.44; k = 3) and 36% (SD = 0.21; k = 2) of participants, respectively.
Intervention Characteristics
The intervention setting was most often a clinic (71%); some studies reported delivering the intervention at clinic and community sites (10%) or prison (5%). (The intervention setting was not identified in three studies.) Interventions were conducted over a median of 5 sessions (range = 1 to 16) of 60 minutes each (range = 16 to 143). The intervention was most often delivered in-person (91%; 9% facilitated or delivered entirely by computer/technology) using individual only (55%), group only (23%), or both individual and group sessions (23%). Facilitators delivered group-based interventions to a median of 12 participants. The intervention was typically led by a single facilitator (range = 1 to 2); facilitators were most often paraprofessionals (41%; e.g., counselors).
Most interventions were theory-based (77%); intervention content was based on the transtheoretical model (32%), information-motivation-behavioral skills model (14%), or social cognitive/social learning theory (14%). Study authors also reported using motivational interviewing (32%) or motivational enhancement therapy (18%) techniques to deliver the intervention. The intervention content varied widely but often provided education (73%; 68% HIV, 45% alcohol, 36% sex, 27% medication adherence), personalized alcohol feedback (59%), and skills training (55%; 45% self-management skills; 41% communication skills, 14% condom skills, and 9% alcohol skills); encouraged the identification of risky situations (55%; e.g., drinking before sex); and assisted participants with goal setting (e.g., plans to reduce drinking; 86%).
Control Conditions
The control conditions included active comparisons (55%; e.g., education, time-match alternative intervention) or an assessment-only control (45%). Active comparisons were delivered over a median of 4 sessions (range = 1 to 15) with each session lasting a median of 60 minutes (range = 20 to 135).
Methodological Quality
The studies satisfied an average of 66% (SD = 11%) of the methodological quality (MQ) criteria; total MQ scores ranged from 7 to 20 out of a possible 25 points (M = 16.43, SD = 2.87). All of the study authors described the nature and purpose of the study and used a study design appropriate to test the stated hypotheses. Few studies reported that the participants were representative of the population from which they were recruited (5%) or that they randomly sampled potential participants (24%). Random assignment of individuals or groups to an intervention or control group was most often reported (90%); few studies (10%) reported that participants were blind to their assigned group. Control groups were typically compared (or matched) with the intervention group to determine (or ensure) equivalency (95%). Standardized treatment by using a manual and/or providing specific training to facilitators was reported in 90% of the studies. All of the studies reported the use of valid and reliable measures to assess the main outcomes; only 10 studies reported including an objective outcomes (e.g., blood; 48%). Only 14% reported blinding those measuring the intervention outcomes. Most studies (76%) used an assessment that occurred six months or later post-intervention. Intervention compliance was reported in most studies (76%). Retention was high with 90% of the studies reporting that at least 70% of the sample completed the study. Withdrawals or dropped outs were described in most studies (62%). Few studies (33%) considered missing data in their outcome reporting (e.g., intent-to-treat, compared with non-attrition cases at baseline); 52% of the studies used statistical methods that controlled for baseline and/or other characteristics. There were no differences for any of the outcomes based on the proportion of MQ criteria satisfied [results not shown].
Impact of the Interventions Compared with Controls
The weighted mean effect sizes and homogeneity statistics for the between-group analyses at the last assessment are presented in Table II. These analyses are presented separately by type of outcome: alcohol use, sexual risk behaviors, and antiretroviral adherence. (Only 10% of the effect sizes calculated were estimated; analyses excluding the estimated effect sizes [results not shown] revealed the same pattern of results for all outcomes and thus, the estimated effect sizes were retained in the analyses.) All studies provided at least one assessment of alcohol use; assessments of sexual risk behaviors and adherence were provided by 13 and 7 studies, respectively.
Table II.
Weighted mean effect sizes and homogeneity statistics for between-groups analyses at the last assessment.
| Outcome | k | d+ random(95% CI) | Q | p | I2 (95% CI) |
|---|---|---|---|---|---|
| Alcohol Use | |||||
| Alcohol use (% participants) | 10 | 0.04 (−0.15, 0.23) | 66.90 | <.001 | 87 (77, 92) |
| Frequency of drinking days | 5 | 0.18 (−0.16, 0.51) | 14.76 | .005 | 73 (32, 89) |
| Quantity of alcohol consumeda | 11 | 0.11 (0.03, 0.20) | 8.73 | .558 | 0 (0, 74) |
| Heavy drinking | 3 | 0.24 (0.07, 0.41) | 0.26 | .877 | 0 (0, 85) |
| Sexual Risk Behaviors | |||||
| Sexual partners | 4 | 0.09 (−0.10, 0.23) | 4.91 | .427 | 0 (0, 87) |
| Condom use | 11 | 0.24 (0.07, 0.40) | 48.76 | <.001 | 79 (64, 88) |
| Sexual risk composite | 3 | 0.11 (−0.10, 0.33) | 1.81 | .405 | 0 |
| Antiretroviral Adherence | |||||
| Plasma viral load | 7 | 0.14 (0.02, 0.26) | 5.78 | .448 | 0 (0, 56) |
| Medication adherencea | 6 | 0.14 (0.07, 0.21) | 0.98 | .964 | 0 (0, 31) |
Note. Weighted mean effect sizes (d+) are positive for differences that favor the intervention group relative to the control group. K, number of interventions; CI, confidence interval; Q, homogeneity statistic; p, probability value for the homogeneity statistic; I2, consistency of effect sizes.
Two outliers were detected for the quantity of alcohol consumed (44, 46) and a single outlier was detected for self-reports of medication adherence (44). The magnitude and direction of the weighted mean effect sizes that included the outlier(s) was generally consistent with the above outcomes (quantity of alcohol consumed: d+ = −0.08, 95% CI = −0.35, 0.19, k = 13; medication adherence: d+ = 0.18, 95% CI = 0.08, 0.27, k = 7).
Alcohol use
Intervention participants reduced their quantity of alcohol consumed (d+ random = 0.11, 95% CI = 0.03, 0.20; k = 11) and reported less heavy drinking (d+ random = 0.24, 95% CI = 0.07, 0.41; k = 3) relative to control conditions. The hypothesis of homogeneity was supported for both the quantity of alcohol consumed (p = .558) and heavy drinking (p =.877), but the uncertainty limits were wide and exceeded the 50% threshold. There were no significant differences between the intervention and control participants on alcohol use or the frequency of drinking days. The hypothesis of homogeneity for alcohol consumption (p < .001) or the frequency of drinking days (p = .005) was not supported.
Sexual risk behaviors
Intervention participants increased their condom use compared to controls (d+ random = 0.24, 95% CI = 0.07, 0.40; k = 11). The hypothesis of homogeneity was not supported (Q [12] = 48.76, p <.001; I2 = 79, 95% CI = 64, 88). There were no significant differences between the intervention and control groups with respect to the number of sexual partners or composite indices of sexual risk.
Antiretroviral adherence
Intervention participants had significant reductions in their plasma viral load relative to controls (d+ random = 0.14, 95% CI = 0.02, 0.26; k = 7). The hypothesis of homogeneity was supported (p = .448) but the uncertainty limits of the I2 were wide (range = 0 to 56) and exceeded the 50% threshold. Medication adherence increased among intervention participants relative to controls (d+ random = 0.14, 95% CI = 0.07, 0.21; k = 6). The hypothesis of homogeneity was supported (Q [5] = 0.98, p = .964; I2 = 0, 95% CI = 0, 31).
Comparison of Single-Behavior vs. Multiple-Behavior Interventions
Interventions were more successful at reducing the frequency of drinking days when the intervention targeted alcohol use alone (d+ random = 0.56, 95% CI = 0.28, 0.83; k = 2) vs. alcohol use and other HIV-related behaviors (d+ random = −0.06, 95% CI = −0.26, −0.14; k = 3), QB (1) = 12.57, p <.001. No significant differences were found for the proportion of participants who drank alcohol or the quantity of alcohol consumed. (The impact of the type of intervention could not be assessed for heavy drinking, sexual risk behaviors, and adherence as only a single study for each outcome evaluated an alcohol-only intervention.)
Moderators
Moderator tests were conducted to examine whether hypothesized moderators of intervention efficacy including study (geographical region, recruitment setting), sample (proportion women, proportion problematic alcohol use, and proportion currently on ART), or intervention length (total intervention dose) related to the variability in the between-group effect sizes. Due to insufficient sample size (k ≤ 5), moderator tests were conducted only for the following outcomes: alcohol use, frequency of drinking days, quantity consumed, condom use, plasma viral load, and medication adherence.
Only a few moderators of intervention efficacy were identified for two outcomes—percent using alcohol and condom use (see Table III). Compared to controls, interventions were more successful in reducing the proportion of participants who consumed alcohol if the study recruited patients from a clinic setting (vs. other/mixed settings) (QB [1] = 10.25, p <.001). Interventions (vs. controls) were less successful in increasing condom use if the intervention was delivered for longer durations (B = −.00, SE = .00, p =.019). There were no significant differences on any other test for moderation.
Table III.
Moderator analyses for behavioral outcomes stratified by study, sample, or intervention characteristics
| % Alcohol Use | Frequency of Drinking Days | Quantity of Alcohol Consumed | Condom Use | Medication Adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CONTINUOUS VARIABLES | |||||||||||
| k | B (SE) | k | B (SE) | k | B (SE) | k | B (SE) | k | B (SE) | ||
| Women (%) | 10 | −0.17 (0.25) | 4 | 0.58 (0.31) | 10 | −0.21 (0.20) | 11 | −0.02 (0.25) | 7 | −0.14 (0.19) | |
| Tau-squared | .0485 | .0334 | .0000 | .0559 | .0070 | ||||||
| I2 | 80% | 44% | 0% | 81% | 31% | ||||||
| Problem drinkers (%) | 6 | −0.17 (0.16) | 0 | – | 9 | −0.08 (0.17) | 6 | −0.25 (0.24) | 6 | 0.06 (0.11) | |
| Tau-squared | .0115 | .0485 | .0000 | .0344 | .0000 | ||||||
| I2 | 44% | 80% | 0% | 72% | 0% | ||||||
| Antiretroviral therapy (%) | 9 | −0.29 (0.46) | 3 | 2.63 (0.88) | 8 | 0.25 (0.18) | 6 | 0.38 (0.58) | 7 | −0.22 (0.29) | |
| Tau-squared | .0647 | .0086 | .0000 | .0571 | .0048 | ||||||
| I2 | 87% | 14% | 0% | 83% | 26% | ||||||
| Intervention dose | 9 | −0.00 (0.00) | 4 | 0.00 (0.00) | 9 | −0.00 (0.00) | 8 | −0.00 (0.00) | 6 | −0.00 (0.00) | |
| Tau-squared | .0198 | .2782 | .0000 | .0000 | .0086 | ||||||
| I2 | 49% | 84% | 0% | 0% | 19% | ||||||
| CATEGORICAL VARIABLES | |||||||||||
| k |
d+ random (95% CI) |
k |
d+ random (95% CI) |
k |
d+ random (95% CI) |
k |
d+ random (95% CI) |
k |
d+ random (95% CI) |
||
| Geographical Location | |||||||||||
| Americas | 7 | −0.01 (−0.18, 0.17) | 3 | 0.21 (−0.27, 0.70) | 8 | 0.15 (0.03, 0.27) | 8 | 0.24 (0.02, 0.46) | 6 | 0.13 (−0.00, 0.27) | |
| Other locations | 3 | 0.12 (−0.15, 0.39) | 2 | 0.12 (−0.50, 0.74) | 3 | 0.07 (−0.06, 0.19) | 3 | 0.24 (−0.10, 0.57) | 1 | 0.00 (−0.66, 0.66) | |
| QB (1) | 0.56 | 0.05 | 0.99 | 0.00 | 0.05 | ||||||
| p | .454 | .817 | .319 | .974 | .816 | ||||||
| Recruitment setting | |||||||||||
| Clinic only | 6 | 0.17 (0.04, 0.31) | 4 | 0.23 (−0.21, 0.68) | 8 | 0.10 (0.01, 0.20) | 7 | 0.31 (0.11, 0.51) | 4 | 0.19 (0.04, 0.34) | |
| Other settings | 4 | −0.15 (−.30, −.00) | 1 | 0 (−0.81, 0.81) | 3 | 0.13 (−0.04, 0.31) | 4 | 0.10 (−0.16, 0.36) | 3 | 0.19 (0.01, 0.39) | |
| QB (1) | 10.25 | – | 0.11 | 1.64 | 0.00 | ||||||
| p | .001 | .743 | .200 | .989 | |||||||
Assessment of Risk of Bias
Risk of bias was assessed for the three outcomes (alcohol use, quantity of alcohol consumed, and condom use) with 10 or more effect sizes. Funnel plots and results of the statistical tests appear in the Supplementary Materials (S2). The graphical and statistical tests revealed no asymmetries that might be interpreted as small-study effects for alcohol use, quantity of alcohol consumed, or condom use.
DISCUSSION
Our meta-analysis of 21 studies evaluating interventions targeting alcohol use for 8,461 PLWHA found that interventions were successful in reducing alcohol consumption. Importantly, interventions with a significant alcohol focus also increased condom use and medication adherence among PLWHA. Furthermore, improvements in medication adherence among intervention participants were corroborated by parallel changes in plasma viral load. Improvements in adherence and plasma viral load observed in this meta-analysis are similar in magnitude and direction to those found in a prior meta-analysis directly evaluating single-targeted medication adherence interventions (adherence: odds ratio [OR] = 1.50, d = 0.26 vs. d+ = 0.14; viral load: OR = 1.25, d = 0.14 vs. d+ = 0.14) (56). Thus, adding alcohol content does not appear to decrease the efficacy of adherence interventions and may have the added benefit of reducing risky drinking. Therefore, alcohol interventions for PLWHA also improve behaviors that are critical for reducing HIV transmission and improving HIV care.
Overall, very few interventions have targeted alcohol use among PLWHA (2, 6, 57). Only four studies in this meta-analysis focused exclusively on alcohol use (16, 38, 42, 52), whereas the majority of the studies addressed alcohol use as part of a multiple HIV behavior change intervention. The standard of care for HIV often involves addressing all risky health behaviors (58) but it has been unclear whether focused interventions (i.e., those targeting a single behavioral target such as alcohol use) are more effective than those targeting multiple behaviors (e.g., alcohol use and risky sex) simultaneously (10). The results of this meta-analysis suggest that interventions targeting alcohol use alone were more successful in reducing the frequency of drinking days relative to interventions addressing multiple HIV-related behaviors, but there were no difference between the type of intervention used (i.e., single vs. multiple) when we assessed the quantity of alcohol use. Thus, a single health behavior change approach may be more effective in reducing alcohol use among PLWHA but additional comparative efficacy research is needed.
Overall, the tests for moderation identified few moderators of intervention efficacy despite the substantial heterogeneity found in some of the study outcomes (e.g., condom use, see Table II). Two exceptions to this overall pattern were observed: (a) Interventions recruiting patients from a clinic setting (vs. other/mixed settings) tended to be more successful in reducing the proportion of participants who drank alcohol at follow-up. This finding is consistent with the evidence that alcohol screening and brief interventions among clinic patients can reduce alcohol consumption and avert adverse health consequences associated with alcohol use among PLWHA as well as the broader population in primary care (59, 60); and (b) Interventions delivered over longer durations were less effective in increasing condom use. Many of the interventions included in this meta-analysis were lengthy (median = 8 hours), which could have increased fatigue in a population already coping with a number of HIV-related problems. Although somewhat counter-intuitive, this finding is consistent with the broader literature showing that brief interventions can be more effective than interventions of longer durations in some contexts (61).
Limitations
The findings should be interpreted in light of the limitations of the meta-analysis. First, study inclusion criteria restricted the set of studies to only those that addressed alcohol use as part of the intervention and assessed (or reported) an alcohol use outcome. Second, most outcomes involve self-reports, which are vulnerable to measurement, cognitive (e.g., memory), and social (e.g., self-presentation) biases (62). Third, there were too few studies of single and multiple behavior targets to identify the efficacy of each in terms of sexual risk behavior and medication adherence. Future research should compare single versus multiple target interventions on a range of outcomes for PLWHA. Fourth, moderator tests were limited to the data available in the primary-level studies and, therefore, some moderator tests could not be completed due to the low number of cases (e.g., recruitment setting among studies assessing the frequency of drinking days) or incomplete information (e.g., proportion of time spent addressing alcohol). Furthermore, we could not assess whether the efficacy of the interventions was based on the type, technique, or content given the variability of the interventions (e.g., motivational interviewing for alcohol use, secondary HIV prevention, cognitive behavioral stress management to increase medication adherence, family-based intervention for mothers living with HIV). Future research should identify the specific intervention types, techniques, and components that increase the efficacy of the intervention to lower alcohol consumption, reduce sexual risk behaviors, and improve medication adherence. Finally, inconsistencies in the measuring of problematic alcohol use as well as the reporting of clinical and immunological markers (e.g., baseline CD4 counts, viral load) prevented us from fully exploring potential moderators that may explain these findings.
CONCLUSION
Few studies have targeted alcohol use among PLWHA despite the potential benefits. This may be due, in part, to the prioritization of other challenges (e.g., medication adherence, HIV-related stigma, mental health concerns), limited clinic time, competing life stressors, or other barriers. Nonetheless, interventions targeting alcohol use among PLWHA are efficacious in reducing high-risk alcohol, sexual, and nonadherent behaviors that are known to be associated with secondary HIV transmission and poor clinical outcomes. Continued development, testing, and refinement of interventions to reduce alcohol use among PLWHA is needed, as are strategies to integrate alcohol interventions into clinical care and the stressful life circumstances of PLWHA.
Supplementary Material
Acknowledgments
Funding: Research reported in this paper was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R01AA021355 to Lori A. J. Scott-Sheldon, PhD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: All authors declare that they have no conflicts of interest.
Ethical Approval: This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Joint United Nations Programme on HIV/AIDS. Global AIDS Update. UNAIDS; 2016. Contract No.: January 16. [PubMed] [Google Scholar]
- 2.Scott-Sheldon LAJ, Walstrom P, Carey KB, Johnson BT, Carey MP, The Mash Research Team Alcohol Use and Sexual Risk Behaviors among Individuals Infected with HIV: A Systematic Review and Meta-Analysis 2012 to Early 2013. Current HIV/AIDS reports. 2013;10(4):314–23. doi: 10.1007/s11904-013-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: Results from the HIV Cost and Services Utilization Study. Journal of studies on alcohol. 2002;63(2):179–86. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 4.Center for Behavioral Health Statistics and Quality. (HHS Publication No SMA 16-4984, NSDUH Series H-51).Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. 2016 [Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015/NSDUH-FFR1-2015/NSDUH-FFR1-2015.pdf.
- 5.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sexually Transmitted Diseases. 2007;34(11):856–63. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 6.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcoholism, clinical and experimental research. 2016;40(10):2056–72. doi: 10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J. Causal Considerations on Alcohol and HIV/AIDS: A Systematic Review. Alcohol and Alcoholism. 2010;45(2):159–66. doi: 10.1093/alcalc/agp091. [DOI] [PubMed] [Google Scholar]
- 8.Samet JH, Walley AY. Interventions targeting HIV-infected risky drinkers: Drops in the bottle. Alcohol Research & Health. 2010;33(3):267–79. [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JL, DeMartini KS, Sales JM, Swartzendruber AL, DiClemente RJ. Interventions to reduce alcohol use among HIV-infected individuals: a review and critique of the literature. Current HIV/AIDS Reports. 2013;10(4):356–70. doi: 10.1007/s11904-013-0174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noar SM, Chabot M, Zimmerman RS. Applying health behavior theory to multiple behavior change: considerations and approaches. Preventive Medicine. 2008;46(3):275–80. doi: 10.1016/j.ypmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska JJ, Prochaska JO. A Review of Multiple Health Behavior Change Interventions for Primary Prevention. American journal of lifestyle medicine. 2011;5(3) doi: 10.1177/1559827610391883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology & Community Health. 1998;52(6):377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. British Medical Journal. 1991;302(6785):1136–40. doi: 10.1136/bmj.302.6785.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WR, Brown JM, Simpson TL, Handmaker NS, Bien TH, Luckie LF. What works? A methodological analysis of the alcohol treatment outcome literature. In: Hester RK, Miller WR, editors. Handbook of alcoholism treatment approaches: Effective alternatives. 2nd. Needham Heights, MA: Allyn & Bacon; 1995. pp. 12–44. [Google Scholar]
- 16.Chander G, Hutton HE, Lau B, Xu X, McCaul ME. Brief intervention decreases drinking frequency in HIV-infected, heavy drinking women: Results of a randomized controlled trial. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;70(2):137–45. doi: 10.1097/QAI.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, McPhee SJ, et al. Interactive “Video Doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3(4):e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velasquez MM, von Sternberg K, Johnson DH, Green C, Carbonari JP, Parsons JT. Reducing sexual risk behaviors and alcohol use among HIV-positive men who have sex with men: A randomized clinical trial. Journal of consulting and clinical psychology. 2009;77(4):657–67. doi: 10.1037/a0015519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of Clinical Epidemiology. 2001;54(10):1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Borenstein M. Software for Publication Bias. In: Rothstein H, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments West Sussex. United Kingdom: Wiley; 2005. [Google Scholar]
- 24.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. New York: Erlbaum; 1998. [Google Scholar]
- 26.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological methods. 2002;7(1):105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BT, Eagly AH. Meta-Analysis of Research in Social and Personality Psychology. In: Reis HT, Judd CM, editors. Handbook of research methods in social and personality psychology. 2nd. London: Cambridge University Press; 2014. pp. 675–707. [Google Scholar]
- 28.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 29.Sanchez-Meca J, Marin-Martinez F, Chacon-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychological Methods. 2003;8(4):448–67. doi: 10.1037/1082-989X.8.4.448. [DOI] [PubMed] [Google Scholar]
- 30.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics. 1981;6:107–28. [Google Scholar]
- 31.StataCorp. Stata/SE 12.1 for Windows. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 32.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata. 2001 [Google Scholar]
- 33.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedges LV. Fixed effects models. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 285–99. [Google Scholar]
- 37.Bachanas P, Kidder D, Medley A, Pals SL, Carpenter D, Howard A, et al. Delivering Prevention Interventions to People Living with HIV in Clinical Care Settings: Results of a Cluster Randomized Trial in Kenya, Namibia, and Tanzania. AIDS and behavior. 2016 doi: 10.1007/s10461-016-1349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasin DS, Aharonovich E, O’Leary A, Greenstein E, Pavlicova M, Arunajadai S, et al. Reducing heavy drinking in HIV primary care: A randomized trial of brief intervention, with and without technological enhancement. Addiction. 2013;108(7):1230–40. doi: 10.1111/add.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jean PC. The influence of psychological predictors and cognitive behavioral stress management intervention on antiretroviral therapy (ART) adherence among HIV-positive female Haitian alcohol users [PhD] Ann Arbor: Florida International University; 2015. [Google Scholar]
- 40.Naar-King S, Parsons JT, Murphy DA, Chen XG, Harris DR, Belzer ME. Improving Health Outcomes for Youth Living With the Human Immunodeficiency Virus A Multisite Randomized Trial of a Motivational Intervention Targeting Multiple Risk Behaviors. Archives of Pediatrics & Adolescent Medicine. 2009;163(12):1092–8. doi: 10.1001/archpediatrics.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naar-King S, Wright K, Parsons JT, Frey M, Templin T, Lam P, et al. Healthy choices: Motivational enhancement therapy for health risk behaviors in HIV-positive youth. AIDS Education and Prevention. 2006;18(1):1–11. doi: 10.1521/aeap.2006.18.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, et al. Treatment outcomes of a stage 1 cognitive–behavioral trial to reduce alcohol use among human immunodeficiency virus-infected out-patients in Western Kenya. Addiction. 2011;106(12):2156–66. doi: 10.1111/j.1360-0443.2011.03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: A randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes. 2007;46(4):443–50. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rongkavilit C, Naar-King S, Wang B, Panthong A, Bunupuradah T, Parsons JT, et al. Motivational interviewing targeting risk behaviors for youth living with HIV in Thailand. AIDS and behavior. 2013;17(6):2063–74. doi: 10.1007/s10461-013-0407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rotheram-Borus MJ, Rice E, Comulada WS, Best K, Elia C, Peters K, et al. Intervention outcomes among HIV-affected families over 18 months. AIDS and behavior. 2012;16(5):1265–75. doi: 10.1007/s10461-011-0075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samet JH, Horton NJ, Meli S, Dukes K, Tripps T, Sullivan L, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antiviral therapy [Internet] 2005;10(1):83–93. doi: 10.1177/135965350501000106. [DOI] [PubMed] [Google Scholar]
- 47.Samet JH, Raj A, Cheng DM, Blokhina E, Bridden C, Chaisson CE, et al. HERMITAGE—A randomized controlled trial to reduce sexually transmitted infections and HIV risk behaviors among HIV-infected Russian drinkers. Addiction. 2015;110(1):80–90. doi: 10.1111/add.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senyonyi RM, Underwood LA, Suarez E, Musisi S, Grande TL. Cognitive behavioral therapy group intervention for HIV transmission risk behavior in perinatally infected adolescents. Health (1949–4998) 2012;4(12):1334–45. [Google Scholar]
- 49.Sikkema KJ, Hansen NB, Kochman A, Santos J, Watt MH, Wilson PA, et al. The development and feasibility of a brief risk reduction intervention for newly HIV-diagnosed men who have sex with men. Journal of Community Psychology. 2011;39(6):717–32. doi: 10.1002/jcop.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sikkema KJ, Wilson PA, Hansen NB, Kochman A, Neufeld S, Ghebremichael MS, et al. Effects of a coping intervention on transmission risk behavior among people living with HIV/AIDS and a history of childhood sexual abuse. Journal of acquired immune deficiency syndromes (1999) 2008;47(4):506–13. doi: 10.1097/QAI.0b013e318160d727. [DOI] [PubMed] [Google Scholar]
- 51.Sorensen JL, Dilley J, London J, Okin RL, Delucchi KL, Phibbs CS. Case management for substance abusers with HIV/AIDS: A randomized clinical trial. The American journal of drug and alcohol abuse. 2003;29(1):133–50. doi: 10.1081/ada-120018843. [DOI] [PubMed] [Google Scholar]
- 52.Wandera B, Tumwesigye NM, Nankabirwa JI, Mafigiri DK, Parkes-Ratanshi RM, Kapiga S, et al. Efficacy of a Single, Brief Alcohol Reduction Intervention among Men and Women Living with HIV/AIDS and Using Alcohol in Kampala, Uganda: A Randomized Trial. Journal of the International Association of Providers of AIDS Care. 2016 doi: 10.1177/2325957416649669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss SM, Tobin JN, Antoni M, Ironson G, Ishii M, Vaughn A, et al. Enhancing the health of women living with HIV: the SMART/EST Women’s Project. International journal of women’s health. 2011;3:63–77. doi: 10.2147/IJWH.S5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zack B, Grinstead O, Faigeles B. A health promotion intervention for prison inmates with HIV. In: Bowser BP, Mishra SI, Reback CJ, Lemp GF, Bowser BP, Mishra SI, et al., editors. Preventing AIDS: Community-science collaborations. New York, NY, US: Haworth Press; 2004. pp. 97–114. [Google Scholar]
- 55.World Health Organization. Global burden of disease: Definitions of region groupings. 2013 [Available from: http://www.who.int/about/regions/en/index.html.
- 56.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of Interventions in Improving Highly Active Antiretroviral Therapy Adherence and HIV-1 RNA Viral Load: A Meta-Analytic Review of Randomized Controlled Trials. Journal of acquired immune deficiency syndromes (1999) 2006;43(01):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. Journal of Acquired Immune Deficiency Syndromes. 2009;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention. Incorporating HIV prevention into the medical care of persons living with HIV. Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2003;52:1–24. [PubMed] [Google Scholar]
- 59.Bertholet N, Daeppen J, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: Systematic review and meta-analysis. Archives of Internal Medicine. 2005;165(9):986–95. doi: 10.1001/archinte.165.9.986. [DOI] [PubMed] [Google Scholar]
- 60.Savage CL, Sanchez M. Alcohol and Substance Use Disorder Screening, Brief Intervention, and Referral to Treatment Among People Living With HIV/AIDS. J Addict Nurs. 2016;27(3):214–7. doi: 10.1097/JAN.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 61.Johnson BT, Michie S, Snyder LB. Effects of Behavioral Intervention Content on HIV Prevention Outcomes: A Meta-Review of Meta-Analyses. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;66:S259–S70. doi: 10.1097/QAI.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: II. Accuracy of self-reports. Annals of Behavioral Medicine. 2003;26(2):104–23. doi: 10.1207/s15324796abm2602_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Orden OR. The influence of event-level factors and processes of change on safe sex and alcohol use among HIV+ men who have sex with men [PhD] Ann Arbor: University of Maryland, Baltimore County; 2013. [Google Scholar]
- 64.Kidder DP, Bachanas P, Medley A, Pals S, Nuwagaba-Biribonwoha H, Ackers M, et al. HIV Prevention in Care and Treatment Settings: Baseline Risk Behaviors among HIV Patients in Kenya, Namibia, and Tanzania. Plos One. 2013;8(2) doi: 10.1371/journal.pone.0057215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bachanas P, Medley A, Pals S, Kidder D, Antelman G, Benech I, et al. Disclosure, Knowledge of Partner Status, and Condom Use Among HIV-Positive Patients Attending Clinical Care in Tanzania, Kenya, and Namibia. AIDS Patient Care & STDs. 2013;27(7):425–35. doi: 10.1089/apc.2012.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medley A, Seth P, Pathak S, Howard AA, Deluca N, Matiko E, et al. Alcohol use and its association with HIV risk behaviors among a cohort of patients attending HIV clinical care in Tanzania, Kenya, and Namibia. AIDS Care – Psychological and Socio-Medical Aspects of AIDS/HIV. 2014;26(10):1288–97. doi: 10.1080/09540121.2014.911809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seth P, Kidder D, Pals S, Parent J, Mbatia R, Chesang K, et al. Psychosocial functioning and depressive symptoms among HIV-positive persons receiving care and treatment in Kenya, Namibia, and Tanzania. Prevention science: the official journal of the Society for Prevention Research [Internet] 2014;15(3):318–28. doi: 10.1007/s11121-013-0420-8. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/225/CN-01047225/frame.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antelman G, Medley A, Mbatia R, Pals S, Arthur G, Haberlen S, et al. Pregnancy desire and dual method contraceptive use among people living with HIV attending clinical care in Kenya, Namibia and Tanzania. J Fam Plann Reprod Health Care. 2015;41(1):e1. doi: 10.1136/jfprhc-2013-100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerbert B, Berg-Smith S, Mancuso M, Caspers N, McPhee S, Null D, et al. Using innovative video doctor technology in primary care to deliver brief smoking and alcohol intervention. Health Promotion Practice. 2003;4(3):249–61. doi: 10.1177/1524839903004003009. [DOI] [PubMed] [Google Scholar]
- 70.Gerbert B, Danley DW, Herzig K, Clanon K, Ciccarone D, Gilbert P, et al. Refraining ‘Prevention with Positives’: Incorporating Counseling Techniques That Improve the Health of HIV-Positive Patients. AIDS Patient Care and STDs. 2006;20(1):19–29. doi: 10.1089/apc.2006.20.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong FL, Rotheram-Borus MJ, Lightfoot M, Pequegnat W, Comulada WS, Cumberland W, et al. Effects of behavioral intervention on substance use among people living with HIV: The healthy living project randomized controlled study. Addiction. 2008;103(7):1206–14. doi: 10.1111/j.1360-0443.2008.02222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elliott JC, Aharonovich E, O’Leary A, Wainberg M, Hasin DS. Drinking motives among HIV primary care patients. AIDS and behavior. 2014;18(7):1315–23. doi: 10.1007/s10461-013-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elliott JC, Aharonovich E, O’Leary A, Wainberg M, Hasin DS. Drinking motives as prospective predictors of outcome in an intervention trial with heavily drinking HIV patients. Drug and Alcohol Dependence. 2014;134(1):290–5. doi: 10.1016/j.drugalcdep.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aharonovich E, Stohl M, Ellis J, Amrhein P, Hasin D. Commitment strength, alcohol dependence and healthcall participation: effects on drinking reduction in HIV patients. Drug and Alcohol Dependence. 2014;135:112–8. doi: 10.1016/j.drugalcdep.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elliott JC, Aharonovich E, Hasin DS. Reasons for limiting drinking in an HIV primary care sample. Alcoholism: Clinical and Experimental Research. 2014;38(6):1720–7. doi: 10.1111/acer.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elliott JC, Aharonovich E, O’Leary A, Johnston B, Hasin DS. Perceived medical risks of drinking, alcohol consumption, and hepatitis C status among heavily drinking hiv primary care patients. Alcoholism: Clinical and Experimental Research. 2014;38(12):3052–9. doi: 10.1111/acer.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elliott JC, Aharonovich E, Hasin DS. Post-treatment drinking among HIV patients: Relationship to pre-treatment marijuana and cocaine use. Drug and Alcohol Dependence. 2015;151:115–20. doi: 10.1016/j.drugalcdep.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elliott JC, Delker E, Wall MM, Feng T, Aharonovich E, Tracy M, et al. The Importance of Context: Neighborhood Drinking Norms and Heavy Drinking Among HIV Patients. Journal of acquired immune deficiency syndromes (1999) 2016;72(2):e55–7. doi: 10.1097/QAI.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elliott JC, Delker E, Wall MM, Feng T, Aharonovich E, Tracy M, et al. Neighborhood-level drinking norms and individual-level drinking among HIV-infected heavy drinkers. Alcoholism: Clinical and Experimental Research. 2016;40:108A. doi: 10.1111/acer.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gause NK, Elliott JC, Delker E, Stohl M, Hasin D, Aharonovich E. Association between change in self-efficacy to resist drinking and drinking behaviors among an HIV-infected sample: Results from a large randomized controlled trial. Journal of health psychology. 2016 doi: 10.1177/1359105316664127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elliott JC, Stohl M, Aharonovich E, O’Leary A, Hasin DS. Reasons for drinking as predictors of alcohol involvement one year later among HIV-infected individuals with and without hepatitis C. Annals of Medicine. 2016:1–7. doi: 10.1080/07853890.2016.1206668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naar-King S, Kolmodin K, Parsons JT, Murphy D. Psychosocial factors and substance use in high-risk youth living with HIV: A multi-site study. AIDS Care. 2010;22(4):475–82. doi: 10.1080/09540120903220279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Outlaw A, Naar-King S, Janisse H, Parsons JT, Adolescent Trials Network HA Predictors of condom use in a multisite study of high-risk youth living with HIV. Aids Education and Prevention. 2010;22(1):1–14. doi: 10.1521/aeap.2010.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nugent NR, Brown LK, Belzer M, Harper GW, Nachman S, Naar-King S. Youth living with HIV and problem substance use: elevated distress is associated with nonadherence and sexual risk. Journal of the International Association of Physicians in AIDS Care (JIAPAC) 2010;9(2):113–5. doi: 10.1177/1545109709357472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naar-King S, Parsons JT, Murphy D, Kolmodin K, Harris DR. A multisite randomized trial of a motivational intervention targeting multiple risks in youth living with HIV: initial effects on motivation, self-efficacy, and depression. Journal of Adolescent Health. 2010;46(5):422–8. doi: 10.1016/j.jadohealth.2009.11.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanney MR, Naar-King S, Murphy DA, Parsons JT, Janisse H, Team ATNP Multiple Risk Behaviors Among Youth Living with Human Immunodeficiency Virus in Five US Cities. Journal of Adolescent Health. 2010;46(1):11–6. doi: 10.1016/j.jadohealth.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X, Murphy DA, Naar-King S, Parsons JT. A clinic-based motivational intervention improves condom use among subgroups of youth living with HIV. Journal of Adolescent Health. 2011;49(2):193–8. doi: 10.1016/j.jadohealth.2010.11.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy DA, Chen X, Naar-King S, Parsons for the Adolescent Trials Network JT Alcohol and Marijuana Use Outcomes in the Healthy Choices Motivational Interviewing Intervention for HIV-Positive Youth. AIDS Patient Care & STDs. 2012;26(2):95–1006. doi: 10.1089/apc.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen J, Mattson M, Miller W, Tonigan J, Connors G, Rychtarik R, et al. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of studies on alcohol. 1997;58(1):7–29. [PubMed] [Google Scholar]
- 90.Naar-King S, Lam P, Wang B, Wright K, Parsons JT, Frey MA. Brief report: Maintenance of effects of motivational enhancement therapy to improve risk behaviors and HIV-related health in a randomized controlled trial of youth living with HIV. Journal of pediatric psychology. 2008;33(4):441–5. doi: 10.1093/jpepsy/jsm087. [DOI] [PubMed] [Google Scholar]
- 91.Naar-King S, Wright K, Parsons JT, Frey M, Templin T, Ondersma S. Transtheoretical Model and substance use in HIV-positive youth: Routledge. 2006:839–45. doi: 10.1080/09540120500467075. [DOI] [PubMed] [Google Scholar]
- 92.Naar-King S, Wright K, Parsons JT, Frey M, Templin T, Ondersma S. Transtheoretical model and condom use in HIV-positive youths. Health Psychology. 2006;25(5):648. doi: 10.1037/0278-6133.25.5.648. [DOI] [PubMed] [Google Scholar]
- 93.Papas RK, Gakinya BN, Sidle JE, Martino S, Baliddawa JB, Carroll KM, et al., editors. Alcoholism: Clinical and Experimental Research. 2013. Gender differences in drinking, mood symptoms and risk behaviors among HIV-infected outpatients in western kenya. [Google Scholar]
- 94.Papas RK, Gakinya BN, Baliddawa JB, Martino S, Bryant KJ, Meslin EM, et al. Ethical issues in a stage 1 cognitive-behavioral therapy feasibility study and trial to reduce alcohol use among HIV-infected outpatients in Western Kenya. Journal of Empirical Research on Human Research Ethics. 2012;7(3):29–37. doi: 10.1525/jer.2012.7.3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papas RK, Sidle JE, Martino S, Baliddawa JB, Songole R, Omolo OE, et al. Systematic cultural adaptation of cognitive-behavioral therapy to reduce alcohol use among HIV-infected outpatients in western Kenya. AIDS and behavior [Internet] 2010;14(3):669–78. doi: 10.1007/s10461-009-9647-6. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/452/CN-00752452/frame.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elia C. Talk-LA: An Overview of the Underlying Principles of the Intervention. UCLA Semel Institute Center for Community Health; 2008. [Google Scholar]
- 97.Parsons JT, Rosof E, Mustanski B. Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. Journal of health psychology. 2007;12(2):357–70. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parsons JT, Rosof E, Mustanski B. Medication adherence mediates the relationship between adherence self-efficacy and biological assessments of HIV health among those with alcohol use disorders. AIDS and behavior. 2008;12(1):95–103. doi: 10.1007/s10461-007-9241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parsons JT, Rosof E, Mustanski B. The temporal relationship between alcohol consumption and HIV-medication adherence: a multilevel model of direct and moderating effects. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2008;27(5):628–37. doi: 10.1037/a0012664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Longmire-Avital B, Golub SA, Parsons JT. Self-reevaluation as a critical component in sustained viral load change for HIV+ adults with alcohol problems. Annals of Behavioral Medicine. 2010;40(2):176–83. doi: 10.1007/s12160-010-9194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Longmire-Avital B, Holder CA, Golub SA, Parsons JT. Risk Factors for Drinking among HIV-Positive African American Adults: The Depression-Gender Interaction. American Journal of Drug & Alcohol Abuse. 2012;38(3):260–6. doi: 10.3109/00952990.2011.653425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rongkavilit C, Wang B, Naar-King S, Bunupuradah T, Parsons JT, Panthong A, et al. Motivational interviewing targeting risky sex in HIV-positive young Thai men who have sex with men. Arch Sex Behav. 2015;44(2):329–40. doi: 10.1007/s10508-014-0274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dyer TP, Stein JA, Rice E, Rotheram-Borus MJ. Predicting depression in mothers with and without HIV: The role of social support and family dynamics. AIDS and behavior. 2012;16(8):2198–208. doi: 10.1007/s10461-012-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rice E, Comulada S, Green S, Arnold EM, Rotheram-Borus MJ. Differential disclosure across social network ties among women living with HIV. AIDS and behavior. 2009;13(6):1253–61. doi: 10.1007/s10461-009-9554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lester P, Stein JA, Bursch B, Rice E, Green S, Penniman T, et al. Family-Based Processes Associated with Adolescent Distress, Substance Use and Risky Sexual Behavior in Families Affected by Maternal HIV. Journal of Clinical Child and Adolescent Psychology. 2010;39(3):328–40. doi: 10.1080/15374411003691677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanchez M, Rice E, Stein J, Milburn NG, Rotheram-Borus MJ. Acculturation, coping styles, and health risk behaviors among HIV positive Latinas. AIDS and behavior. 2010;14(2):401–9. doi: 10.1007/s10461-009-9618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rotheram-Borus MJ, Rice E, Comulada WS, Best K, Li L. Comparisons of HIV-affected and non-HIV-affected families over time. Vulnerable children and youth studies. 2012;7(4):299–314. doi: 10.1080/17450128.2012.713532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glover DA, Garcia-Aracena EF, Lester P, Rice E, Rotheram-Borus MJ. Stress biomarkers as outcomes for HIV+ prevention: participation, feasibility and findings among HIV+ Latina and African American mothers. AIDS and behavior. 2010;14(2):339–50. doi: 10.1007/s10461-009-9549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rice E, Lester P, Flook L, Green S, Valladares ES, Rotheram-Borus MJ. Lessons learned from “integrating” intensive family-based interventions into medical care settings for mothers living with HIV/AIDS and their adolescent children. AIDS and behavior. 2009;13(5):1005–11. doi: 10.1007/s10461-008-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elia C. Talk-LA: A Family-Centered Approach to Mental Health. UCLA Center for Community Health; 2008. [Google Scholar]
- 111.Rotheram-Borus MJ, Swendeman D, Lee S-J, Li L, Amani B, Nartey M. Interventions for families affected by HIV. Translational behavioral medicine. 2011;1(2):313–26. doi: 10.1007/s13142-011-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rotheram-Borus MJ, Murphy DA, Miller S, Draimin BH. An intervention for adolescents whose parents are living with AIDS. Clinical Child Psychology and Psychiatry. 1997;2(2):201–19. [Google Scholar]
- 113.Chuang CH, Liebschutz JM, Horton NJ, Samet JH. Association of violence victimization with inconsistent condom use in HIV-infected persons. AIDS and behavior. 2006;10(2):201–7. doi: 10.1007/s10461-005-9046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: Are they related? Alcoholism: Clinical and Experimental Research. 2003;27(5):862–7. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- 115.Palepu A, Raj A, Horton NJ, Tibbetts N, Meli S, Samet JH. Substance abuse treatment and risk behaviors among HIV-infected persons with alcohol problems. Alcoholism-Clinical and Experimental Research. 2005;28(5):162A–A. doi: 10.1016/j.jsat.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 116.Palepu A, Norton NJ, Tibbetts N, Meli S, Samet JH. Substance Abuse Treatment and Hospitalization among a Cohort of HIV-Infected Individuals with Alcohol Problems. Alcoholism: Clinical and Experimental Research. 2005;29(3):389–94. doi: 10.1097/01.alc.0000156101.84780.45. [DOI] [PubMed] [Google Scholar]
- 117.Palepu A, Horton NJ, Tibbetts N, Dukes K, Meli S, Samet JH. Substance abuse treatment and emergency department utilization among a cohort of HIV-infected persons with alcohol problems. Pergamon Press – An Imprint of Elsevier Science; 2003. p. 37. [DOI] [PubMed] [Google Scholar]
- 118.Chuang CH, Liebschutz JM, Cheng DM, Raj A, Samet JH. Substance use during sexual and physical assault in HIV-infected persons. Violence and victims. 2007;22(2):216–25. doi: 10.1891/088667007780477311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paasche-Orlow MK, Cheng DM, Palepu A, Meli S, Faber V, Samet JH. Health literacy, antiretroviral adherence, and HIV-RNA suppression – A longitudinal perspective. Journal of General Internal Medicine. 2006;21(8):835–40. doi: 10.1111/j.1525-1497.2006.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim TW, Kertesz SG, Horton NJ, Tibbetts N, Samet JH. Episodic homelessness and health care utilization in a prospective cohort of HIV-infected persons with alcohol problems. BMC health services research. 2006;6:19. doi: 10.1186/1472-6963-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raj A, Cheng DM, Levison R, Meli S, Samet JH. Sex trade, sexual risk, and nondisclosure of HIV serostatus: findings from HIV-infected persons with a history of alcohol problems. AIDS and behavior. 2006;10(2):149–57. doi: 10.1007/s10461-005-9050-x. [DOI] [PubMed] [Google Scholar]
- 122.Smith KL, Horton NJ, Saitz R, Samet JH. The use of the mini-mental state examination in recruitment for substance abuse research studies. Drug and Alcohol Dependence. 2006;82(3):231–7. doi: 10.1016/j.drugalcdep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 123.Liebschutz JM, Geier JL, Horton NJ, Chuang CH, Samet JH. Physical and sexual violence and health care utilization in HIV-infected persons with alcohol problems. AIDS Care. 2005;17(5):566–78. doi: 10.1080/09540120512331314358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcoholism, clinical and experimental research. 2004;28(4):572–7. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 125.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 2004;99(3):361–8. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 126.Lunze K, Cheng DM, Quinn E, Krupitsky E, Raj A, Walley AY, et al. Nondisclosure of HIV infection to sex partners and alcohol’s role: A Russian experience. AIDS & Behavior. 2013;17(1):390–8. doi: 10.1007/s10461-012-0216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raj A, Kidd JD, Cheng DM, Coleman S, Bridden C, Blokhina EA, et al. Associations between partner violence perpetration and history of STI among HIV-infected substance using men in Russia. AIDS Care. 2013;25(5):646–51. doi: 10.1080/09540121.2012.722188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tyurina A, Krupitsky E, Cheng DM, Coleman SM, Walley AY, Bridden C, et al. Is cannabis use associated with HIV drug and sex risk behaviors among Russian HIV-infected risky drinkers? Drug and Alcohol Dependence. 2013;132(1–2):74–80. doi: 10.1016/j.drugalcdep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsui JI, Cheng DM, Coleman SM, Lira MC, Blokhina E, Bridden C, et al. Pain is associated with risky drinking over time among HIV-infected persons in St. Petersburg, Russia. Drug and Alcohol Dependence. 2014;144:87–92. doi: 10.1016/j.drugalcdep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pace C, Lioznov D, Cheng D, Wakeman S, Raj A, Walley A, et al. Sexually transmitted infections among HIV-infected heavy drinkers in St Petersburg, Russia. International journal of STD & AIDS. 2012;23(12):853–8. doi: 10.1258/ijsa.2012.012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goodness TM, Palfai TP, Cheng DM, Coleman SM, Bridden C, Blokhina E, et al. Depressive symptoms and antiretroviral therapy (ART) initiation among HIV-infected Russian drinkers. AIDS and behavior. 2014;18(6):1085–93. doi: 10.1007/s10461-013-0674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palfai TP, Cheng DM, Coleman SM, Bridden C, Krupitsky E, Samet JH. The influence of depressive symptoms on alcohol use among HIV-infected Russian drinkers. Drug and Alcohol Dependence. 2014;134:85–91. doi: 10.1016/j.drugalcdep.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Urada LA, Raj A, Cheng DM, Quinn E, Bridden C, Blokhina EA, et al. History of intimate partner violence is associated with sex work but not sexually transmitted infection among HIV-positive female drinkers in Russia. International Journal of STD & AIDS. 2013;24(4):287–92. doi: 10.1177/0956462412472809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kiriazova T, Cheng D, Coleman S, Blokhina E, Krupitsky E, Lira M, et al. Factors associated with study attrition among HIV-infected risky drinkers in St. Petersburg, Russia. HIV clinical trials. 2014;15(3):116–25. doi: 10.1310/hct1503-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blokhina E, Krupitsky E, Cheng D, Raj A, Walley A, Bridden C, et al. Polysubstance use among HIV-infected drinkers in Russia. European Neuropsychopharmacology. 2011;21:S167. [Google Scholar]
- 136.Edelman EJ, Cheng DM, Krupitsky EM, Bridden C, Quinn E, Walley AY, et al. Heroin Use and HIV Disease Progression: Results from a Pilot Study of a Russian Cohort. AIDS and behavior. 2014:1–9. doi: 10.1007/s10461-014-0948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walley AY, Cheng DM, Coleman SM, Krupitsky E, Raj A, Blokhina E, et al. Risk factors for recent nonfatal overdose among HIV-infected Russians who inject drugs. AIDS Care. 2014;26(8):1013–8. doi: 10.1080/09540121.2013.871218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Samet J, Lunze K, Cheng DM, Lioznov D, Quinn E, Bridden C, et al. HIV-related stigma and substance use in a Russian cohort of HIV+ risky drinkers. Drug and alcohol dependence [Internet] 2015;156:e197. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/702/CN-01134702/frame.html. [Google Scholar]
- 139.Senyonyi RM. CBT Group Counseling Intervention for HIV Transmission Risk Behavior in Perinatally Infected Adolescents [PhD] Ann Arbor: Regent University; 2012. [Google Scholar]
- 140.Carrico AW, Chesney MA, Johnson MO, Morin SF, Neilands TB, Remien RH, et al. Randomized controlled trial of a cognitive-behavioral intervention for HIV-positive persons: an investigation of treatment effects on psychosocial adjustment. AIDS and behavior. 2009;13(3):555–63. doi: 10.1007/s10461-008-9429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meade CS, Hansen NB, Kochman A, Sikkema KJ. Utilization of medical treatments and adherence to antiretroviral therapy among HIV-positive adults with histories of childhood sexual abuse. AIDS Patient Care and STDs. 2009;23(4):259–66. doi: 10.1089/apc.2008.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sikkema KJ, Hansen NB, Meade CS, Kochman A, Fox AM. Psychosocial predictors of sexual HIV transmission risk behavior among HIV-positive adults with a sexual abuse history in childhood. Arch Sex Behav. 2009;38(1):121–34. doi: 10.1007/s10508-007-9238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meade CS, Drabkin AS, Hansen NB, Wilson PA, Kochman A, Sikkema KJ. Reductions in alcohol and cocaine use following a group coping intervention for HIV-positive adults with childhood sexual abuse histories. Addiction. 2010;105(11):1942–51. doi: 10.1111/j.1360-0443.2010.03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Persons E, Kershaw T, Sikkema KJ, Hansen NB. The impact of shame on health-related quality of life among HIV-positive adults with a history of childhood sexual abuse. AIDS Patient Care STDS. 2010;24(9):571–80. doi: 10.1089/apc.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sikkema KJ, Ranby KW, Meade CS, Hansen NB, Wilson PA, Kochman A. Reductions in traumatic stress following a coping intervention were mediated by decreases in avoidant coping for people living with HIV/AIDS and childhood sexual abuse. Journal of consulting and clinical psychology. 2013;81(2):274–83. doi: 10.1037/a0030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilson SM, Sikkema KJ, Ranby KW. Gender moderates the influence of psychosocial factors and drug use on HAART adherence in the context of HIV and childhood sexual abuse. AIDS Care. 2014;26(8):959–67. doi: 10.1080/09540121.2013.873765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Willie TC, Overstreet NM, Peasant C, Kershaw T, Sikkema KJ, Hansen NB. Anxiety and Depressive Symptoms Among People Living with HIV and Childhood Sexual Abuse: The Role of Shame and Posttraumatic Growth. AIDS and behavior. 2016;20(8):1609–20. doi: 10.1007/s10461-016-1298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Willie TC, Overstreet NM, Sullivan TP, Sikkema KJ, Hansen NB. Barriers to HIV Medication Adherence: Examining Distinct Anxiety and Depression Symptoms among Women Living with HIV Who Experienced Childhood Sexual Abuse. Behavioral medicine (Washington, DC) 2016;42(2):120–7. doi: 10.1080/08964289.2015.1045823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schwartz B, Dilley J, Sorensen J. Case management of substance abusers with HIV disease. Journal of case management. 1993;3(4):173–8. [PubMed] [Google Scholar]
- 150.Masson CL, Sorensen JL, Phibbs CS, Okin RL. Predictors of medical service utilization among individuals with co-occurring HIV infection and substance abuse disorders. AIDS Care – Psychological and Socio-Medical Aspects of AIDS/HIV. 2004;16(6):744–55. doi: 10.1080/09540120412331269585. [DOI] [PubMed] [Google Scholar]
- 151.Parsons JT, Kutnick AH, Halkitis PN, Punzalan JC, Carbonari JP. Sexual Risk Behaviors and Substance Use Among Alcohol Abusing HIV-Positive Men Who Have Sex With Men. Journal of Psychoactive Drugs. 2005;37(1):27–36. doi: 10.1080/02791072.2005.10399746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parsons JT, Huszti HC, Crudder SO, Rich LM, Mendoza J. Maintenance of safer sexual behaviours: evaluation of a theory-based intervention for HIV seropositive men with haemophilia and their female partners. Haemophilia. 2000;6(3):181–90. doi: 10.1046/j.1365-2516.2000.00404.x. [DOI] [PubMed] [Google Scholar]
- 153.Parsons JT, Vicioso KJ, Punzalan JC, Halkitis PN, Kutnick A, Velasquez MM. The Impact of Alcohol Use on the Sexual Scripts of HIV-Positive Men Who Have Sex With Men. Journal of Sex Research. 2004;41(2):160–72. doi: 10.1080/00224490409552224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lopez E, Jones DL, Ishii M, Tobin JN, Weiss SM. HIV medication adherence and substance use: The Smartest Women’s Project. American journal of infectious diseases. 2007;3(4):240. doi: 10.3844/ajidsp.2007.240.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jones DL, McPherson-Baker S, Lydston D, Camille J, Brondolo E, Tobin JN, et al. Efficacy of a group medication adherence intervention among HIV positive women: the SMART/EST Women’s Project. AIDS and behavior. 2007;11(1):79–86. doi: 10.1007/s10461-006-9165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Segal-Isaacson CJ, Tobin JN, Weiss SM, Brondolo E, Vaughn A, Wang C, et al. Improving Dietary Habits in Disadvantaged Women With HIV/AIDS: The SMART/EST Women’s Project. AIDS and behavior. 2006;10(6):659–70. doi: 10.1007/s10461-006-9115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Grinstead O, Zack B, Faigeles B. Reducing postrelease risk behavior among HIV seropositive prison inmates: The health promotion program. AIDS Education & Prevention. 2001;13(2):109–19. doi: 10.1521/aeap.13.2.109.19737. [DOI] [PubMed] [Google Scholar]
- 158.Rothman AJ, Salovey P. Shaping perceptions to motivate healthy behavior: the role of message framing. Psychological bulletin. 1997;121(1):3. doi: 10.1037/0033-2909.121.1.3. [DOI] [PubMed] [Google Scholar]
- 159.Miller WR, Rollnick S. Motivational interviewing: Helping people change. Guilford press; 2012. [Google Scholar]
- 160.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. American journal of health promotion. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 161.Fisher JD, Fisher WA. The information-motivation-behavioral skills model. Emerging theories in health promotion practice and research: Strategies for improving public health. 2002;1:40–70. [Google Scholar]
- 162.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 163.Strecher VJ, Champion VL, Rosenstock IM. The health belief model and health behavior. In: Gochman DS, editor. Handbook of Health Behavior Research I: Personal and Social Determinants. New York: Plenum Press; 1997. pp. 71–91. [Google Scholar]
- 164.Miller WR, Zweben A, DiClemente CC, Rychtarik R. Motivational Enhancement Therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: National Institute of Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- 165.Antoni MH, Baggett L, Ironson G, LaPerriere A, August S, Klimas N, et al. Cognitive-behavioral stress management intervention buffers distress responses and immunologic changes following notification of HIV-1 seropositivity. Journal of consulting and clinical psychology. 1991;59:906. doi: 10.1037//0022-006x.59.6.906. [DOI] [PubMed] [Google Scholar]
- 166.Babor TF, Higgins-Biddle JC, Saunders JB, Moneiro MG. AUDIT: The Alcohol Use Disorders Identification Test. World Health Organization; 2001. [Available from: http://www.talkingalcohol.com/files/pdfs/WHO_audit.pdf. [Google Scholar]
- 167.Ewing JA. Detecting alcoholism. The CAGE questionnaire. Journal of the American Medical Association. 1984;252(14):1905–7. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


