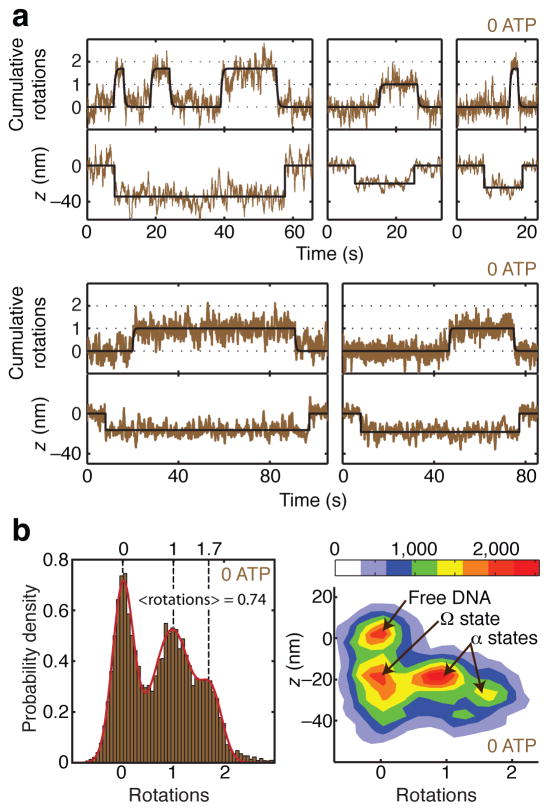
Figure 4.
Distinct conformations of the nucleotide-free complex. (a) Measurements of angle and z during gyrase binding events in the absence of ATP, showing transitions between three distinct contracted states. Contraction initially occurs without rotation, and the rotor subsequently makes long-lived excursions to ~1 rotation and ~1.7 rotations. (b) Histograms of angle and z during gyrase binding events in the absence of ATP, comprising 41 reversible excursions. For the 1D histogram, only portions of the rotor trace showing z contraction were selected for analysis. Solid line shows a fit to the sum of three Gaussians centered at ~0 rotations, ~1 rotation, and ~1.7 rotations. The mean angle is 0.74 ± 0.07 rotations (error estimated as SEM of waiting angles based on N=87 dwells). For the 2D histogram of paired (angle, z) data, z contraction events were included along with 2000 frames (8 s) of flanking data on either side of each event, yielding a distinct population corresponding to enzyme-free DNA. The newly identified Ω state, which is contracted in z but does not trap supercoils, can be distinguished from states (designated α) that trap positive supercoils. The legend shows the correspondence between color coding and histogram counts, where each count represents a data point from a 4 ms frame.