FIGURE 3.
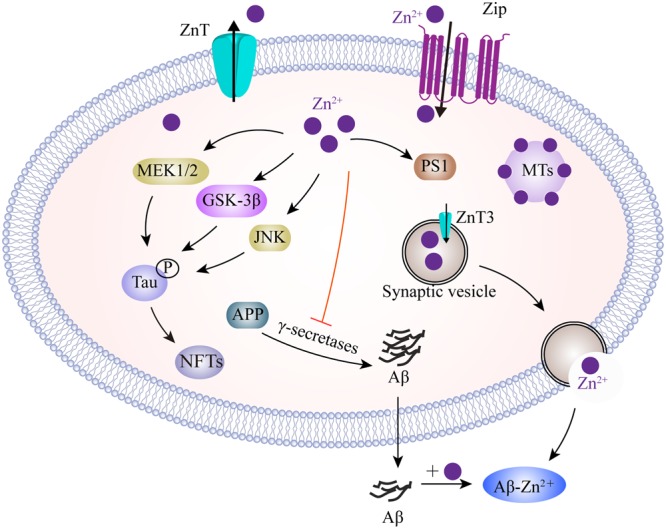
A model describing the zinc transport system and zinc imbalance in the AD brain. Zn2+ enters neurons, mainly depending on ZIPs, whereas Zn2+ efflux is controlled by ZnT in the plasma membrane. Intracellularly, MTs as the major Zn2+-buffering peptides, maintain Zn2+ at appropriate levels. In glutamatergic neurons, Zn2+ is transported into presynaptic vesicles by ZnT3. Thus, Zn2+ could be co-released with glutamate into the synaptic cleft during neuronal activity. Zn2+ binds to Aβ and promotes its aggregation. Increased Zn2+ enhances tau translation and phosphorylation by MEK1/2, GSK3β and JNK pathways. In addition, Zn2+ increases APP proteolysis, but inhibits γ-secretase activity. Zn2+ is also involved in the upregulation of PS1, which facilitates cellular Zn2+ uptake. ZIPs, zinc importing proteins; ZnT, zinc transporter; MTs, metallothioneins; MEK1/2, mitogen-activated protein kinase 1/2; GSK3β, glycogen synthase kinase 3β; JNK, c-Jun N-terminal kinase; PS1, presenilin 1.
