Table 2.
Scope of the asymmetric [3+1]-cycloaddition reaction of enoldiazoacetate 1 with sulfur ylides 2.[a]
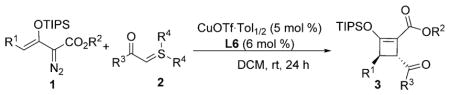
| ||||
|---|---|---|---|---|
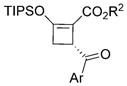
| ||||
| Yield(%)[b] | ee(%)[c] | |||
| 3a[e,f]: | 72 | 83 | (R2 = Me, Ar = Ph, R4 = Ph) | |
| 3b: | 81 | 72 | (R2 = Me, Ar = 4-ClC6H4, R4 = Ph) | |
| 3c: | 82 | 70 | (R2 = Me, Ar = 4-BrC6H4, R4 = Ph) | |
| 3d: | 73 | 73 | (R2= tBu, Ar = Ph, R4 = Ph) | |
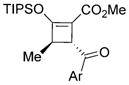
| ||||
| Yield(%)[b] | ee(%)[c] | d.r.[d] | ||
| 3e: | 74 | 93 | >20:1 | (Ar = Ph, R4 = Me) |
| 3f: | 80 | 93 | 16:1 | (Ar = 4-MeC6H4, R4 = Me) |
| 3g: | 82 | 98 | 14:1 | (Ar = 4-MeOC6H4, R4 = Me) |
| 3h: | 72 | 99 | >20:1 | (Ar = naphthyl, R4 = Me) |
| 3i: | 80 | 95 | 18:1 | (Ar = 4-ClC6H4, R4 = Me) |
| 3j: | 74 | 97 | >20:1 | (Ar = 4-BrC6H4, R4 = Me) |
| 3k: | 86 | 96 | 15:1 | (Ar = 2-thiophenyl, R4 = Me) |
| 3l: | 58 | 97 | 16:1 | (Ar = 4-CNC6H4, R4 = Ph) |
Reaction conditions: 1 (0.24 mmol, 1.2 equiv) in dry DCM (1.0 mL) was added to a 1.0 mL DCM solution of 2 (0.20 mmol, 1.0 equiv), CuOTf·Tol1/2 (0.01 mmol), and L6 (0.012 mmol) under N2 within 1 h.
Yield of isolated product 3 based on the limiting reagent 2.
Enantiomeric excesses determined by HPLC analysis with a chiral stationary phase.
Diastereomeric ratios were determined by 1H NMR analysis of the reaction mixtures.
Reactions performed at −20°C.
Reactions performed for 48 hours.
