Abstract
Krüppel-like factors (KLFs) belong to the zinc finger family of transcription factors and their function in the CNS is largely unexplored. KLF11 is a member of the KLF family and we have previously demonstrated that peroxisome proliferator-activated receptor gamma-mediated cerebral protection during ischemic insults needs recruitment of KLF11 as its critical coactivator. Here we sought to determine the role of KLF11 itself in cerebrovascular function and the pathogenesis of ischemic stroke. Transient middle cerebral artery occlusion (MCAO) was performed in KLF11 knockout and wild-type control mice, and brain infarction was analyzed by TTC staining. BBB integrity was assessed by using Evans Blue and TMR-Dextran extravasation assays. KLF11 KO mice exhibited significantly larger brain infarction and poorer neurological outcomes in response to ischemic insults. Genetic deficiency of KLF11 in mice also significantly aggravated ischemia-induced BBB disruption by increasing cerebrovascular permeability and edema. Mechanistically, KLF11 was found to directly regulate IL-6 in the brains of ischemic mice. These findings suggest that KLF11 acts as a novel protective factor in ischemic stroke. Elucidating the functional importance of KLF11 in ischemia may lead us to discover novel pharmacological targets for the development of effective therapies against ischemic stroke.
Keywords: Krüppel-like factor 11, Brain infarction, Blood-brain barrier, inflammation, cerebral ischemia
Introduction
Ischemic stroke results from a transient or permanent local reduction of cerebral blood flow, and ranks as the fifth leading cause of death and the leading cause of adult disability in the United States [1,2]. Currently, thrombolytic therapy within a narrow time window is the only acute therapeutic intervention for ischemic stroke and development of effective therapies is urgently required [3,4]. Stroke activates a series of cellular and molecular signaling cascades that eventually lead to ischemic neuronal death in the brain [5,6]. However, the underlying mechanisms of stroke-induced pathophysiological events are not completely understood.
Dysfunction of transcription factors plays an essential role in the pathogenesis of many neurological diseases, including stroke. Up to now, an increasing number of transcription factors and their related gene signaling networks have been demonstrated in the regulation of ischemic brain injury and neurovascular recovery in rodent experimental stroke models [7–9]. Krüppel-like factors (KLFs) belong to the zinc finger family of transcription factors and consist of 17 members with diverse regulatory functions in cell growth, differentiation, proliferation, migration, apoptosis, metabolism, and inflammation [10,11]. Accumulative studies have documented their involvement in various human diseases including cancer, diabetes, obesity, cardiovascular diseases, and inflammatory conditions[12].
KLFs are also abundantly expressed in the central nervous system. But very little is known about their role in brain neural cells associated with central nervous system development and neurological disorders[13]. One research group has reported that KLFs may play a role in regulating intrinsic axon growth ability in primary retinal ganglion cells and cortical neuron cultures since KLF4 and -9 suppressed while KLF6 and -7 increased neurite growth. However, these findings did not show that the KLF family had an effect on neuronal survival[14]. Our laboratory, together with another research team, is among the first to determine the role of KLFs in cerebrovascular function and the pathogenesis of ischemic stroke[15,16]. In a recent publication[16], we screened for PPARγ coregulators using a genome-wide and high-throughput coactivation system and revealed KLF11 as a novel PPARγ coregulator, which was further confirmed to physically interact with PPARγ and regulate PPARγ-mediated cerebrovascular protection in primary brain microvascular endothelial cell cultures and mouse brain after in vitro and in vivo ischemic insults. Another group also investigated the role of KLF2 in the pathogenesis of ischemic stroke, and identified KLF2 as a novel stroke-protective factor in the cerebrovasculature through its regulation of BBB function[15]. However, the role and molecular control of the KLF family in the etiology of ischemic stroke is still in its infancy.
KLF11 is a unique diabetes-associated KLF transcription factor among 17 KLF family members[17,18] and highly expressed in vascular endothelium[19]. Mutations in the KLF11 gene result in Maturity Onset Diabetes of the Young 7 (MODY7), and are closely involved in human type 2 diabetes mellitus[17,18], a major risk factor for stroke[20,21]. Previously, we reported for the first time that PPARγ-mediated cerebrovascular protection during ischemic insults needs recruitment of KLF11 as its critical coactivator, suggesting KLF11 as a potential mediator in stroke pathologies[16]. However, the functional significance and mechanisms of KLF11 itself in regulating cerebrovascular pathogenesis are totally unknown in ischemic stroke.
In this study, we have shown that KLF11 expression is significantly decreased in the cerebral cortex of mice following focal cerebral ischemia. Of note, KLF11 genetic deficiency results in a larger brain infarction, increased BBB permeability/leakage, higher water content, worsened neurobehavioral performance, and less CBF perfusion in mouse ischemic brain regions after middle cerebral artery occlusion. Further, we have shown that KLF11 plays anti-inflammatory roles in mouse brains following ischemic stroke. These results suggest that KLF11 itself plays critical protective roles in ischemic stroke.
Material and methods
All procedures using laboratory animals were approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Stroke Treatment and Academic Industry Roundtable (STAIR) guidelines were adhered to throughout all animal studies. Animals were randomly assigned to various experimental groups using a lottery box. All stroke outcome assessments were performed in a blinded manner.
Mouse model of transient focal cerebral ischemia
Focal cerebral ischemia was induced in male KLF11 knockout mice (kindly provided by Dr. Eugene Chen [16]) and wild-type mice on C57BL/6J background (8–10w, 23–25g) by intraluminal middle cerebral artery occlusion (MCAO) as described previously [22,23,13]. Briefly, mice were anesthetized with 1.5–3% isoflurane (Henry Schein Animal Health). A 2-cm length of a 6–0 rounded tip nylon suture (Doccol Corporation, Sharon, MA) was gently advanced from the internal carotid artery up to the origin of the middle cerebral artery (MCA) until regional cerebral blood flow (CBF) was reduced to less than 25% of baseline. After 1h of MCA occlusion, blood flow was restored by removing the suture, and the mice were allowed to recover for 1–7 days. In sham-operated mice, the same surgical procedure was performed but no suture insertion. Changes in CBF, mean arterial pressure, and heart rate were monitored in animals 15 min before, during, and after MCAO. Animals that did not show a CBF reduction of at least 75% over baseline levels were excluded from further experimentation. Approximate 90% survival rate was observed in KLF11 knockout or wild-type mice at 1–7 d after MCAO. Animals that died after ischemia induction were also excluded. The rectal temperature was controlled at 37.0 ± 0.5°C during surgery.
Assessment of infarct volume, neurological deficit, and sensorimotor function
Mice were sacrificed 24h after MCAO, and infarct area was measured from brain slices using 2% TTC staining as described previously [22,23,13]. Mouse brains were removed and sliced into eight coronal sections (1-mm thick) by a mouse brain matrix. Slices were stained with 2% TTC for 15 min at 37°C and scanned. The infarct area was measured by National Institutes of Health ImageJ software and infarct volume was calculated using a derived formula [22,23,13,24].
Neurobehavioral deficits were determined by the adhesive tape removal test, foot fault test, and rotarod test in mice 1–3 days before MCAO surgery and also at 1, 3, 5 and 7 d of reperfusion after MCAO [25,26]. Following cerebral ischemia, mice were also tested for neurological deficits and scored on a 5-point scale [22,23,13]: 0, no observable neurological deficits (normal); 1, failure to extend right forepaw (mild); 2, circling to the contralateral side (moderate); 3, falling to the right (severe); 4, mice could not walk spontaneously; 5, depressed level of consciousness (very severe).
Quantitation of BBB permeability/leakage
In order to explore the function of KLF11 in regulating cerebral vascular dysfunction after ischemic stroke, we employed two approaches, a classical Evans Blue extravasation assay [22,23] and a fluorescent-labeled Dextran (TMR-Dextran, 70 kDa)[27–29] to examine the change of BBB integrity in KLF11 KO and WT mice subjected to 1h MCAO and 24h reperfusion.
For analysis of cerebrovascular permeability by Evans Blue extravasation, mice were injected with 100 μl of 4% Evans Blue (EB) (Sigma-Aldrich) 23 h after MCAO. One hour later, animals were perfused with 0.9% NaCl and then brains were removed and separated into hemispheres ipsilateral and contralateral to the MCAO. Each hemisphere was then homogenized in N, N-dimethylformamide (Sigma-Aldrich) and centrifuged for 45 min at 25,000 rcf. The supernatants were collected and quantitation of EB extravasation was performed as described [22,23]. Briefly, EB levels in each hemisphere were determined from the formula: (A620nm − ((A500nm + A740nm)/2))/mg wet weight). Background EB levels in the non-ischemic hemisphere were subtracted from the ischemic hemisphere ipsilateral to the MCAO.
For analysis of cerebrovascular permeability by intravenous injection and detection of fluorescent-labeled Dextran, another set of ischemic mice (n=7) were subjected to tail vein injection of Tetramethylrhodamine-Dextran (TMR-Dextran, 70 kDa, 0.1mg/g body weight, Invitrogen, Grand Island, NY, USA) 30 min before the sacrifice time. Animals were anaesthetized and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde in PBS. Brains were collected and cryoprotected in 30% sucrose in PBS, and frozen serial coronal brain sections (30-μm thick) were prepared on a cryostat. Brain sections were visualized directly under a fluorescent microscope. In parallel, brain hemispheres were homogenized in 1% Triton X-100 PBS and fluorescent intensity was quantitated by a fluorometric plate reader using 555 nm excitation and 580 nm emission [27–29].
Quantitative real time PCR
Total RNA was isolated from mouse cerebral cortex by using Trizol (Invitrogen, Carlsbad, CA). Quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR) was carried out with a Bio-Rad CFX Connect Thermocycler, iScript cDNA Synthesis Kit and iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) according to our published protocols [22,30]. Specific primers for 17 KLF members (KLF1–17)or pro-inflammatory factors (IL-6, TNF-α, ICAM-1 and MCP-1) were used for the PCR reaction (Supplemental Table 1). The relative mRNA expression was normalized to cyclophilin RNA levels. PCR experiments were repeated 3 times, each using separate mouse brain samples.
Brain water content
Brain water content was measured by the dry-wet method as described previously[31]. Briefly, mice with strokes were sacrificed by exposure to CO2. The weights of ipsilateral and contralateral hemispheres were recorded separately as wet weights. The dry weights of ipsilateral and contralateral hemispheres were obtained after being heated at 100°C in an oven for 24 hours. Brain content was calculated by the following formula: brain content = (wet weight-dry weight)/wet weight × 100% [31].
Western blot analysis
Total protein from the cerebral cortex was electrophoresed, and transferred to PVDF membranes. The blot was incubated with the following primary antibodies for 1–2 h: mouse anti-KLF2 antibody (1:500; Novus Biologicals, Littleton, CO), rabbit anti-KLF4 antibody (1:500; Novus Biologicals, Littleton, CO), rabbit anti-KLF9 antibody (1:500; Abcam, Cambridge, MA), mouse anti-KLF11 antibody (1:500; Novus Biologicals, Littleton, CO), mouse anti-PPARγ antibody (1:500; Santa Cruz, CA), or mouse anti-actin antiserum (1:500; Santa Cruz, CA). The membrane was then incubated with the secondary antibody (1:5000; anti-rabbit or anti-mouse IgG conjugated with horseradish peroxidase, Promega; Madison, WI) for 1 h, and immunoreactive proteins were visualized by chemiluminescent reagent. The light-emitting bands were detected on X-ray films.
Enzyme-Linked Immunosorbent Assay (ELISA)
Cerebral cortices of mice were collected and sonicated by an ultrasound homogenizer. After centrifugation, supernatant was collected and concentrations of IL-6 and ICAM-1 in brain supernatants from ischemic regions were quantified by Quantikine Mouse IL-6, and ICAM-1 ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. All assays were performed in triplicate.
Laser speckle imaging
A laser speckle imager (Perimed PeriCam PSI HR, Stockholm, Sweden) was used to assess cortical cerebral blood flow (CBF) in mice after cerebral ischemia as described previously[29,32,33]. Mice were anesthetized with 1.5–3% isoflurane and fixed in a head holder in a prone position. The scalp was shaved and cut to expose the thin skull over the bilateral cerebral and cerebellar hemispheres. A CCD camera with a laser speckle imager was placed above the head, and a laser diode (785 nm) illuminated the intact skull surface to allow penetration of the laser in a diffuse manner through the brain. Speckle contrast was used to measure CBF as it is derived from the speckle visibility relative to the velocity of the light-scattering particles (blood). The speckle contrast was then converted to correlation time values, which are inversely proportional to mean blood flow velocity. The mouse was then placed supine, and subjected to 1h MCAO followed by 72h reperfusion. During laser scanning, rectal temperature was controlled at 37°C with a feedback regulated heating pad to minimize data variations due to body temperature. Laser speckle perfusion images were obtained at 15 min before MCAO surgery; 15 min, 30 min, and 60 min during MCAO period; and 15 min, 30 min, 60 min, 2h, 24h, and 72h after the onset of reperfusion. For each animal, five consecutive two-dimension images of the ischemic and nonischemic cerebral hemispheres were scanned at each time point.
For the imaging data analysis, two identical elliptical regions of interest (ROIs) were selected on the ischemic and nonischemic cerebral hemispheres. The blood flow perfusion index was first determined as the ratio of ischemic to non-ischemic cerebral blood flow, and then further normalized to the pre-surgical baseline to obtain the relative CBF value for each animal.
Statistical analysis
Quantitative data are expressed as mean ± SEM. Differences among multiple groups were statistically analyzed by one- or two-way ANOVA followed by a Bonferroni/Dunn post hoc correction. Comparisons between two experimental groups were based on a two-tailed t-test. A p-value less than 0.05 was considered significant.
Results
The expression profiles of 17 KLF family members in mouse brains after focal cerebral ischemia
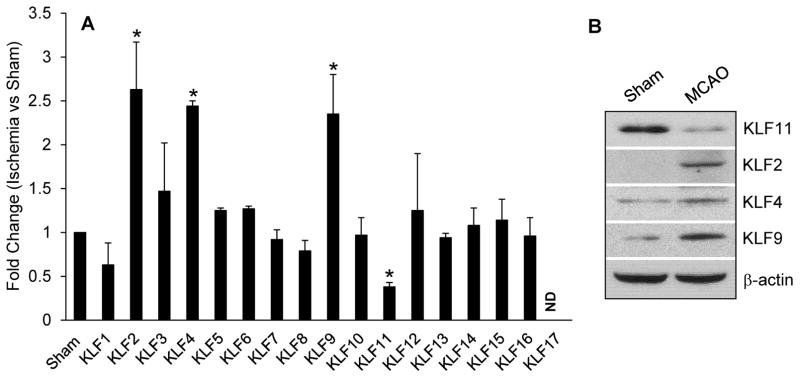
In order to explore the function of KLFs in ischemic neurovascular injury, we first evaluated the expression of 17 cerebral KLFs in a mouse ischemic stroke model. As shown in Figure 1, KLF11 mRNA displayed a ~3-fold reduced expression in the cerebral cortex after 1h MCAO followed by 24h reperfusion. By contrast, the mRNA expression of KLF2, KLF4 and KLF9 was significantly increased following cerebral ischemia (Fig. 1A). Consistently, KLF11 protein level in the ischemic cerebral cortex was significantly decreased while the protein expression of KLF2, KLF4 and KLF9 increased (Fig. 1B). Cerebral ischemia had no effect on other KLF members (Fig. 1). These data documented a dysregulated KLF profile, implying their roles in regulating the pathogenesis of ischemic neurovascular injury.
Fig. 1. Expression profile of 17 KLF family members in mouse cerebral cortex following focal cerebral ischemia.
Cerebral cortex was isolated from C57BL/6J mice after 1h MCAO followed by 24h reperfusion. The mRNA expression profile of 17 KLF family members and the protein level of KLF 2, 4, 9 and 11 were detected by qPCR (A) and Western blotting (B), respectively. Data analysis showed a dramatic decrease of the KLF11 mRNA and protein levels in mouse ischemic regions, whereas three KLF family members: KLF2, KLF4, and KLF9 exhibited significantly increased expression compared with the Sham group (n=3). The other KLF family members had no changes in expression. Data are shown as mean ± SEM. *p<0.05 vs. Sham group.
Genetic deficiency of KLF11 potentiates ischemic brain injury in mice
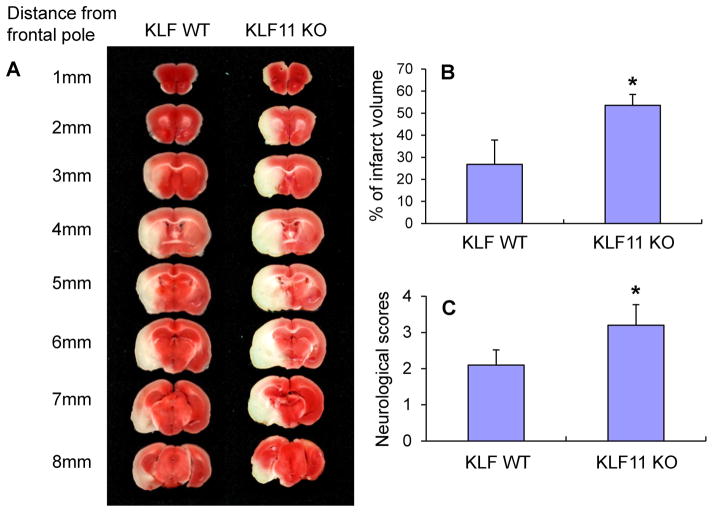
Although KLF11 contributes to vascular endothelial protection[16], it is unknown whether KLF11 itself has any impact on ischemic brain injury in vivo. Thus, we employed an in vivo loss-of-function strategy by using KLF11 knockout (KO) mice on a C57BL/6J background. KLF11 KO mice are viable and fertile with normal appearance, behavior, growth, and litter size. Genotyping PCR showed that genetic deletion of KLF11 has no effect on the expression of the other 16 KLF members in mouse brains (data not shown). KLF11 KO and wild-type control (WT) mice were subjected to transient MCAO for 1h followed by 24h reperfusion. Cerebral infarction was determined by 2% TTC staining and neurological deficits were scored as described in the Methods. In comparison with WT control mice, KLF11 KO mice showed a larger cerebral infarct volume (Fig. 2A–B) and a worsened neurological outcome (Fig. 2C) in response to ischemic insults. These results suggest that loss-of-KLF11 function in mice exacerbates ischemic brain damage.
Fig. 2. Effects of KLF11 genetic deficiency on post-ischemic brain injury.
KLF11 KO mice and WT controls (n=8) were subjected to 1h MCA occlusion and 24h reperfusion. 2% TTC-stained coronal sections (A) were shown at different brain levels from the frontal to the posterior pole. Quantitative analysis was performed on infarct volume (B) and neurological deficits (C) in these mice after ischemic stroke. Genetic deletion of KLF11 robustly increased brain infarct volume and worsened neurological outcomes. Data are shown as mean ± SEM. *p<0.05 vs. WT group.
Genetic deficiency of KLF11 in mice potentiates post-ischemic cerebrovascular permeability
We performed Evans Blue (Fig.3 A–B) [22,23] and TMR-Dextran (Fig.3 C–D) [27–29] extravasation assays to examine and quantify the change of BBB integrity in KLF11 KO and WT mice subjected to 1h MCAO and 24h reperfusion. As shown in Figure 3, genetic deficiency of KLF11 in mice significantly aggravated ischemia-induced BBB disruption by increasing cerebrovascular permeability to either Evans Blue or TMR-Dextran. These results suggest that KLF11 genetic deletion worsens BBB leakage after ischemic stroke.
Fig. 3. Assessment of BBB dysfunction in KLF11 KO mice after focal ischemia.
KLF11 KO mice and WT controls (n=8) were subjected to 1h MCA occlusion and 24h reperfusion. BBB leakage and quantitative analysis were made by Evans Blue (A–B) or 70 kDa TMR-Dextran extravasation (C–D) in mouse brains. Genetic deficiency of KLF11 in mice significantly aggravated ischemia-induced BBB disruption by increasing cerebrovascular permeability to either Evans Blue or TMR-Dextran in comparison with wild-type mice. Data are shown as mean ± SEM. * or #p<0.05 vs. WT or WT+MCAO group.
KLF11 genetic deficiency inhibits neurobehavioral recovery in mice following ischemic stroke
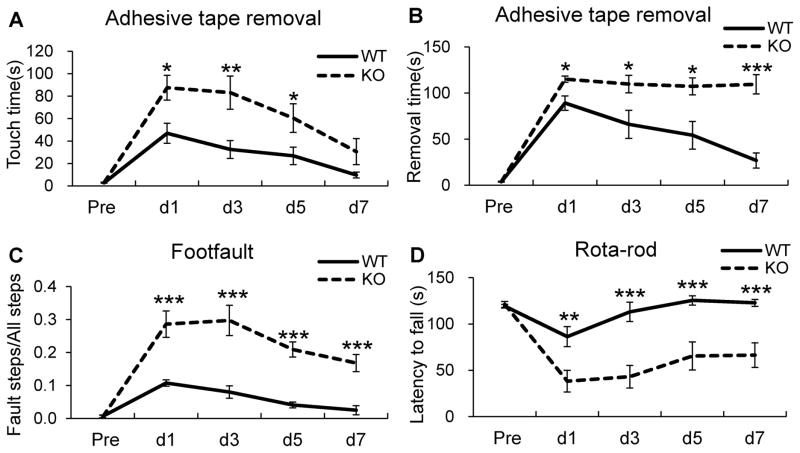
Ischemic stroke in humans often causes extensive sensorimotor dysfunction, and favorable sensorimotor functional recovery is a pivotal step in stroke treatment. As a result, stroke researchers have developed and employed a series of behavioral testing techniques to assess the functional outcomes in experimental stroke models[34,35]. To investigate whether genetic deletion of the KLF11 gene in mice affects post-stroke neurobehavioral function, KLF11 knockout and wild-type control mice were subjected to 1h MCAO followed by 1–7d reperfusion and assessed by three kinds of neurobehavioral tests: adhesive tape removal, foot fault and rotarod tests. Compared to WT control mice, KLF11 KO mice showed robust worsening of sensorimotor function: longer touching or removing time in the adhesive tape removal test (Fig. 4A, B), higher rate of fault steps in the foot fault test (Fig. 4C), and less staying latency in the rotarod test (Fig. 4D). Obviously, KLF11 KO mice exhibited a modest recovery tendency within 7 post-ischemic reperfusion days in comparison with the WT controls. These data demonstrate that KLF11 genetic deficiency leads to a dramatic inhibition of neurobehavioral recovery in mice following ischemic stroke and indicate the vital protective role of the KLF11 gene in response to ischemic stroke.
Fig. 4. Reduced brain sensorimotor functions in KLF11 KO mice.
KLF11 KO mice and WT controls (n=10) were subjected to 1h MCAO followed by 1–7d reperfusion. Sensorimotor deficits were examined by the adhesive tape removal test (A–B), foot fault test (C), and rotarod test (D). We performed all neurobehavioral tests for three consecutive days prior to MCAO surgery as the baseline level, and at every other post-ischemic day until 7 d. Compared to WT controls, mice with KLF11 genetic deletion showed dramatically worsened sensorimotor function (longer touching or removing time in the adhesive tape removal test (A, B), higher rate of fault steps in the foot fault test (C), and reduced staying latency in the rotarod test (D). Data are represented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 vs. WT group.
Genetic deletion of KLF11 enhances brain water content in mice after ischemic stroke
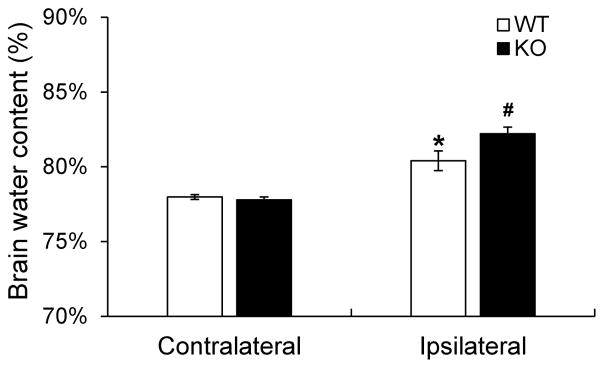
The brain stroke cascade rapidly leads to a chain of neurochemical events that account for irreversible tissue damage, and concurrently, a rapid development of brain edema, which could contribute to even further deterioration of already impaired brain function[36]. In this study, we measured and quantitatively analyzed the water content in both the ipsilateral (ischemic) and contralateral (non-ischemic) hemispheres of KLF11 KO and WT mice 3d following MCAO. As shown in Figure 5, brain water content in the ipsilateral hemisphere of KLF11 KO mice was significantly higher than the WT group (Fig. 5). There is no significant difference in the contralateral hemisphere between these two groups.
Fig. 5. Increased brain water content in KLF11 KO mice after ischemic stroke.
KLF11 KO mice and WT controls were subjected to 1h MCAO and 72 h reperfusion. Brains were dissected out and water content was measured by the wet/dry weight protocol as described in the Methods. Cerebral ischemia caused a significant increase in water content in the ipsilateral but not contralateral hemisphere. Brain water content in KLF11 KO mice was significantly higher than WT controls (n=10). Data are represented as mean ± SEM. *p<0.05: WT ipsilateral hemisphere vs. WT contralateral hemisphere, #p<0.05: KO ipsilateral hemisphere vs. WT ipsilateral hemisphere.
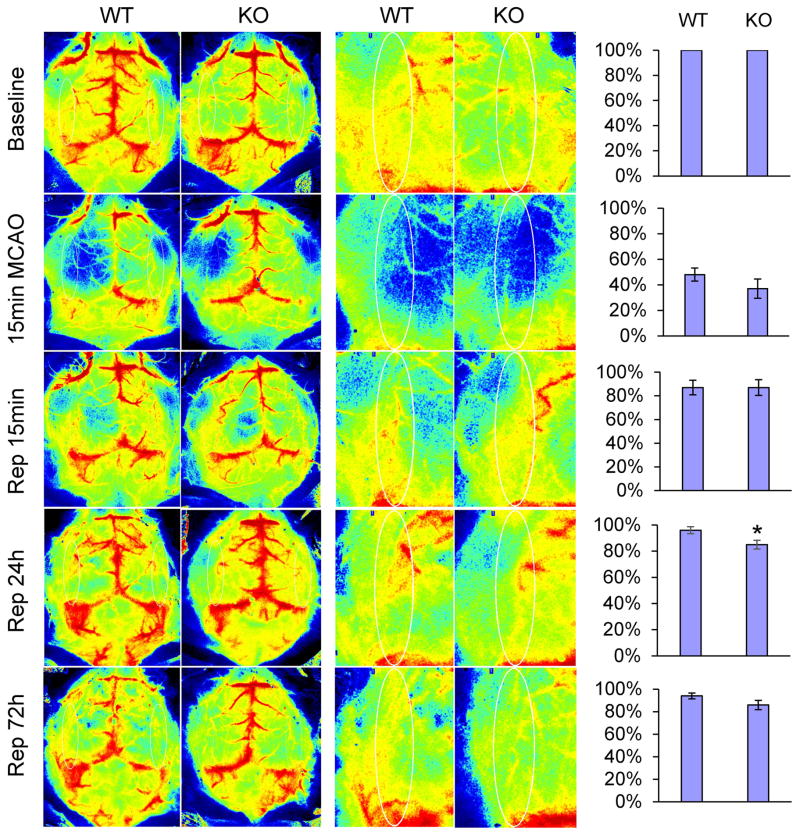
Effects of KLF11 genetic deficiency on CBF reperfusion in mice after ischemic stroke
Cerebral blood flow (CBF) is the blood supply for the brain in a given period of time and determined by a number of elements, such as blood viscosity, dilatory ability of blood vessels, and cerebral perfusion pressure[37]. In this study, we investigated the spatiotemporal changes of CBF in ischemic regions of mice by laser speckle imaging. Two-dimension laser speckle images were scanned and obtained from KLF11 KO and WT mice following cerebral ischemia. As shown in Figure 6, there was no significant difference in the relative CBF value in KLF11 KO mice versus WT mice at baseline, 15 min MCAO, 15 min and 72h reperfusion after 1h MCAO. However, compared with WT controls, KLF11 KO mice revealed a dramatically less CBF recovery at 24h post-ischemic reperfusion, which was consistent with our findings that genetic deficiency of KLF11 exhibited an obviously larger brain infarct volume and a slower neurobehavioral recovery tendency than WT controls following ischemic insult.
Fig. 6. Effects of KLF11 genetic deletion on cerebral blood flow in ischemic brain.
Regional CBF was measured by using a laser speckle imager. KLF11 KO and WT mice were subjected to 1h MCAO followed by 3d reperfusion (n=9). Representative CBF images were shown at 15 min before MCAO (baseline), 15 min after the onset of MCAO, and 15 min, 24h, 72h after the onset of reperfusion. Two identical elliptical ROIs were selected as indicated on the same brain region of the ipsilateral and contralateral hemispheres. The relative CBF was first determined as the ratio of ischemic to non-ischemic cerebral blood flow, and then as the percentage value normalized to the pre-surgical baseline for each animal. Quantitative data analysis demonstrated that the relative CBF value in KLF11 KO mice exhibited a significant decrease at 24h of reperfusion. Data are represented as mean ± SEM. *p<0.05 vs. WT group.
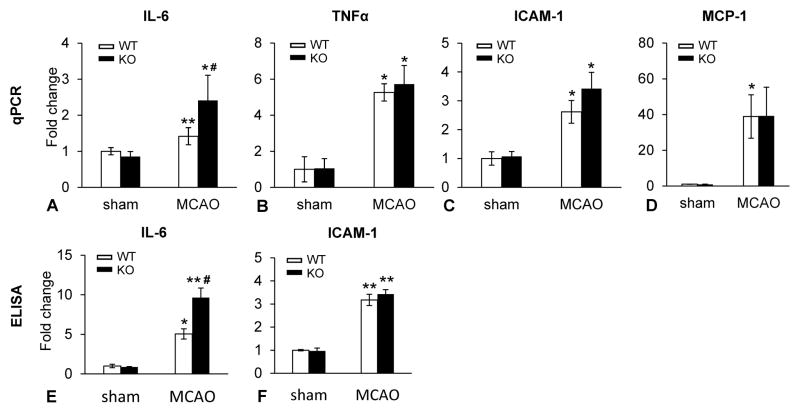
Genetic deletion of KLF11 increases the activities of pro-inflammatory factors in ischemic brain regions
Extensive studies have reported that IL-6 [38], together with other pro-inflammatory cytokines such as TNF-α, ICAM-1 and MCP-1 [39–41], play a part in the pathogenesis of ischemic stroke. To determine if genetic deletion of the KLF11 gene has an impact on cerebral inflammation in mice after ischemic stroke, we assessed the mRNA expression of four well-known pro-inflammatory cytokines: IL-6, TNF-α, ICAM-1 and MCP-1. Our quantitative PCR analysis revealed that brain ischemia induced a significantly increased mRNA production of all four pro-inflammatory factors in the ischemic regions of KLF11 KO or WT mice. Genetic deletion of KLF11 further aggravated ischemia-induced IL-6 but not TNF-α, ICAM-1 or MCP-1 mRNA levels (Fig. 7A–D). We next performed ELISA experiments to examine the activities of the above-mentioned inflammatory factors. Consistent with elevated IL-6 mRNA levels, significantly increased IL-6 activity was found in the ischemic brain regions of KLF11 KO mice in comparison with WT controls (Fig. 7E–G). Taken together, it appears that KLF11 plays cerebrovascular protective roles by specifically inhibiting IL-6 related inflammatory signaling pathways in ischemic stroke.
Fig. 7. Genetic deletion of KLF11 potentiates inflammatory activity in ischemic brain regions.
Stroke was induced in KLF11 KO and WT mice by 1h MCAO followed by 24h reperfusion. Total RNA and protein were extracted from the ipsilateral cerebral cortex 24h after MCAO (n=5). The mRNA expression levels of indicated pro-inflammatory factors were analyzed by qPCR (A–D) and the activities of these inflammatory factors were assessed by ELISA (E–F). Increased mRNA and protein levels of the pro-inflammatory cytokine, IL-6 were found in KLF11 KO mice 24h after MCAO compared with WT controls, whereas several other indicated pro-inflammatory cytokines revealed no significant differences in the ischemic brain regions between the two mouse groups. Experiments were repeated three times. Data are represented as mean ± SEM. *p<0.05 and **p<0.01 vs. KLF11 WT+Sham group, #p<0.05 vs. KLF11 WT+MCAO group.
In addition, to exclude the possibility that KLF11 may play cerebral protective roles through direct regulation of PPARγ, a previously reported cerebrovascular protector[16], as a downstream target, we examined the expression of PPARγ in the ischemic cortex isolated from KLF11 KO and WT mice 1d following MCAO. As showed in Supplemental Figure 1, genetic deletion of KLF11 in mouse did not affect cerebral protein levels of PPARγ in both sham and ischemic conditions, suggesting that KLF11-mediated neuroprotection is solely based on its own property (Supplemental Fig. 1).
Discussion
The present study is the first to characterize the impact of KLF11 genetic deficiency on ischemic brain injury. Genetic deletion of KLF11 in mice resulted in a larger brain infarct volume and increased sensorimotor loss following focal cerebral ischemia. Moreover, KLF11 genetic deficiency significantly aggravated BBB leakage, evidenced by increased Evans Blue and TMR-Dextran extravasation into the brain parenchyma as well as greater water content in mouse ischemic brain regions 3d after MCAO. Furthermore, our data have also shown a significant decrease in regional CBF at 15 min MCAO and dramatically less CBF recovery following 24h of reperfusion in KLF11 KO mice compared to its WT controls. Taken together, our data indicate that KLF11 plays a protective role in the pathogenesis of ischemic stroke.
Currently, accumulative evidence has linked several KLFs to a variety of neurological diseases, including neurodegenerative diseases, epilepsy, CNS carcinoma, stress, depression, alcoholism, neuroinflammation and schizophrenia[13]. However, there are few studies on the role of KLFs in cerebrovascular pathologies following ischemic stroke[13]. Our laboratory, together with other research teams, is among the first to determine the role of KLFs in cerebrovascular function and the pathogenesis of ischemic stroke [15,16]. For example, a research group investigated the role of KLF2, one of the most highly investigated KLF members in endothelial biology, in ischemic stroke. It was demonstrated that ischemia-induced BBB leakage and brain infarction were significantly increased in KLF2 KO mice but markedly decreased in KLF2 transgenic mice following 1h MCAO and 48h reperfusion [15]. Concurrently, in one of our previous publications [16], we examined the role of PPARγ and its coregulators in cerebrovascular endothelial dysfunction after stroke. By using a genome-wide and high-throughput coactivation system, we screened and identified KLF11 as a novel PPARγ coregulator. Moreover, we further found that loss-of-KLF11 function effectively abolished PPARγ agonist pioglitazone-mediated cytoprotection in mouse brain microvascular endothelial cells after in vitro ischemic stimuli. Similarly, cerebrovascular protection derived from PPARγ activation by pioglitazone was also significantly reduced in KLF11 KO mice after MCAO in comparison with its WT controls. Thus, our data clearly indicate that recruitment of KLF11 as a novel PPARγ coregulator is required for PPARγ-mediated cerebrovascular protection in ischemic stroke. However, it is worth noting that in our previous publication, the functional significance and mechanisms of KLF11 itself in regulating cerebrovascular pathogenesis in ischemic stroke were not revealed. In this study, we documented that genetic deletion of KLF11 results in a larger brain infarct size, increased BBB disruption, higher brain edema, worsened neurobehavioral performance, and lower CBF perfusion in mouse ischemic brain regions after ischemic stroke, suggesting KLF11 as a novel stroke-protective transcription factor. Elucidating KLF11 as an endogenous protective mediator may lead us to discover novel pharmacological targets for the development of effective therapies against ischemic stroke.
During cerebral ischemia, inflammatory reactions contribute to the pathogenesis of the disease. Increasing evidence has shown that various brain neural cells are able to mediate cerebrovascular and brain parenchyma inflammation by producing and secreting pro-inflammatory cytokines that initiate primary and delayed neuronal death after cerebral ischemia[42–44]. These pro-inflammatory responses are suggested to be regulated by cerebral KLFs. In this study, we are the first to show that KLF11 plays anti-inflammatory roles in mouse brains after ischemic stroke by its inhibitory effects on IL-6 activity. IL-6 is a major stroke-related pro-inflammatory cytokine[45] and enhanced IL-6 expression contributes to a worsened prognosis and neurological outcomes[46]. Our results are consistent with a previous publication[19] showing that KLF11 is a critical suppressor of endothelial inflammatory activation, and is a novel molecular target for inhibiting vascular inflammatory diseases. Several other studies also reported KLFs’ inflammatory regulatory functions in the brain by showing transcriptional regulation of KLF4, KLF5, and KLF6 on reactive astrogliosis and astrocyte-derived cytokine release after focal or global ischemia[47]. In addition, KLF2 was also found at lower levels in blood outgrowth endothelial cells (BOECs) derived from stroke children with sickle cell anemia (SCA) than in non-SCA BOECs. A downregulated KLF2 level may change the dynamic balance between proinflammatory NF-κB/p65 and anti-inflammatory KLF2, finally leading to a proinflammatory phenotype within the cerebral vasculature and potentially increasing the risk of stroke in children with sickle cell anemia [48]. Collectively, KLF11 regulation of cerebral inflammation represents one of the major mechanisms for its protective role in ischemic stroke.
Cerebral microvascular endothelial damage and resultant BBB dysfunction are major hallmarks of ischemic stroke[49–53,22,16,54,27]. Strategies that enhance BBB stability and reduce BBB leakage hold great promise for the treatment of ischemic stroke[55–57]. Recent studies revealed important roles for the KLF family of transcription factors in endothelial cell biology and pathologies[58,59,13]. However, the functions of KLFs in cerebral endothelial injury and BBB dysfunction after ischemic stroke are poorly understood[15,16,13]. It has been demonstrated that genetic deletion of KLF2 in mice results in an unstable BBB structure and increased stroke-induced BBB dysfunction. Mechanistically, KLF2 is capable of transactivating and upregulating Occludin, a major BBB tight junction protein [15,16,13]. In our study, given the discovery that genetic deletion of KLF11 could obviously augment BBB permeability/leakage and brain water content in mice subjected to MCAO, probing into the contributions and underlying mechanisms of KLF11 in BBB dysfunction after ischemic stroke are worthy topics that should be explored in-depth in the future.
It is well known that multiple mechanisms contribute to post-ischemic BBB breakdown, including but not limited to abnormal pathologies of endothelial tight junctions, loss/impairment of BBB cellular components, inflammation of the vascular wall, increased oxidative stress, and activation of matrix metalloproteinases[49–53,22,16,55–57]. Recently, accumulative studies have uncovered evidence that KLF family members are implicated in pathological vascular processes[58,59], including vascular inflammation. Among them, KLF2 has been reported to regulate endothelial inflammation[60], atherosclerotic progression[61], and endothelial barrier integrity[62]. Another KLF member, KLF4, has been recently identified as an endothelial regulator in response to pro-inflammatory stimuli [63] or shear stress[64], showing vascular anti-inflammatory, anti-atherothrombotic effects in cultured ECs as well as in EC-selective KLF4 transgenic mice[65]. KLF4 was also reported to transactivate VE-cadherin and maintain normal endothelial barrier function[66]. Moreover, in a recent study, the role of KLF11 in the regulation of endothelial inflammation was examined by using intravital fluorescence microscopy[19]. Administration of LPS to KLF11 KO mice increases leukocyte rolling and adhesion on postcapillary venular endothelium compared with WT mice. Also, VCAM-1 and E-selectin levels are significantly increased in aortas from KLF11 KO mice[19]. These results suggest KLF11 represses endothelial inflammation. Based on these previous findings, it is possible that KLF transcription factors may regulate BBB structure and function by mediating BBB tight junction protein levels and controlling cerebrovascular information. In this study, we have not demonstrated the underlying mechanisms for KLF11-mediated BBB protection. Further investigation by utilization of vascular endothelial cell-specific KLF11 transgenic and knockout animals will be necessary to further clarify and provide insight into the mechanisms leading to the pathogenesis of ischemic stroke.
Supplementary Material
Supplementary Table 1: The specific primers for real time PCR
Supplementary Figure 1: Expression of PPARγ protein level in brains of KLF11 KO mice after focal cerebral ischemia. Western blotting data show PPARγ protein level in lysates from cerebral cortex of KLF11 KO and WT mice after 1h MCAO followed by 24h reperfusion. Compared to KLF11 WT controls, there are no significant changes of PPARγ protein levels in brains of KLF11 KO mice at sham or ischemic condition. Representative data are shown from three separate experiments.
Acknowledgments
This work was supported by the National Institutes of Health Grants (NS094930, NS091175, and NS086820 to K.J. Yin).
Footnotes
Disclosure/Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Goldstein LB, Adams R, Becker K, Furberg CD, Gorelick PB, Hademenos G, Hill M, Howard G, Howard VJ, Jacobs B, Levine SR, Mosca L, Sacco RL, Sherman DG, Wolf PA, del Zoppo GJ. Primary prevention of ischemic stroke: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2001;32(1):280–299. doi: 10.1161/01.str.32.1.280. [DOI] [PubMed] [Google Scholar]
- 2.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 3.Schellinger PD, Kaste M, Hacke W. An update on thrombolytic therapy for acute stroke. Curr Opin Neurol. 2004;17(1):69–77. doi: 10.1097/00019052-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Stapf C, Mohr JP. Ischemic stroke therapy. Annual review of medicine. 2002;53:453–475. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- 5.Love S. Apoptosis and brain ischaemia. Progress in neuro-psychopharmacology & biological psychiatry. 2003;27(2):267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 6.Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14(4):469–477. doi: 10.1007/s10495-008-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang YC, Huang CC. Perinatal brain injury and regulation of transcription. Curr Opin Neurol. 2006;19(2):141–147. doi: 10.1097/01.wco.0000218229.73678.a8. [DOI] [PubMed] [Google Scholar]
- 8.Scholzke MN, Schwaninger M. Transcriptional regulation of neurogenesis: potential mechanisms in cerebral ischemia. Journal of molecular medicine. 2007;85(6):577–588. doi: 10.1007/s00109-007-0196-z. [DOI] [PubMed] [Google Scholar]
- 9.Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochemistry international. 2007;50(7–8):1014–1027. doi: 10.1016/j.neuint.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188(2):143–160. doi: 10.1002/jcp.1111. [pii] [DOI] [PubMed] [Google Scholar]
- 11.Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4(2):206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiological reviews. 2010;90(4):1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin KJ, Hamblin M, Fan Y, Zhang J, Chen YE. Krupple-like factors in the central nervous system: novel mediators in Stroke. Metabolic brain disease. 2013 doi: 10.1007/s11011-013-9468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science (New York, NY. 2009;326(5950):298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi H, Sheng B, Zhang F, Wu C, Zhang R, Zhu J, Xu K, Kuang Y, Jameson SC, Lin Z, Wang Y, Chen J, Jain MK, Atkins GB. Kruppel-like factor 2 protects against ischemic stroke by regulating endothelial blood brain barrier function. Am J Physiol Heart Circ Physiol. 2013;304(6):H796–805. doi: 10.1152/ajpheart.00712.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin KJ, Fan Y, Hamblin M, Zhang J, Zhu T, Li S, Hawse JR, Subramaniam M, Song CZ, Urrutia R, Lin JD, Chen YE. KLF11 mediates PPARgamma cerebrovascular protection in ischaemic stroke. Brain. 2013;136(Pt 4):1274–1287. doi: 10.1093/brain/awt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Zapico ME, van Velkinburgh JC, Gutierrez-Aguilar R, Neve B, Froguel P, Urrutia R, Stein R. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J Biol Chem. 2009;284(52):36482–36490. doi: 10.1074/jbc.M109.028852. M109.028852 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, Vaillant E, Benmezroua Y, Durand E, Bakaher N, Delannoy V, Vaxillaire M, Cook T, Dallinga-Thie GM, Jansen H, Charles MA, Clement K, Galan P, Hercberg S, Helbecque N, Charpentier G, Prentki M, Hansen T, Pedersen O, Urrutia R, Melloul D, Froguel P. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102(13):4807–4812. doi: 10.1073/pnas.0409177102. 0409177102[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Y, Guo Y, Zhang J, Subramaniam M, Song CZ, Urrutia R, Chen YE. Kruppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-kappaB signaling pathway. Arterioscler Thromb Vasc Biol. 2012;32(12):2981–2988. doi: 10.1161/ATVBAHA.112.300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen CL, Bayraktutan U. Risk factors for ischaemic stroke. Int J Stroke. 2008;3(2):105–116. doi: 10.1111/j.1747-4949.2008.00187.x. [DOI] [PubMed] [Google Scholar]
- 21.Ringelstein EB, Nabavi D. Long-term prevention of ischaemic stroke and stroke recurrence. Thrombosis research. 2000;98(3):83–96. doi: 10.1016/s0049-3848(00)00230-9. [DOI] [PubMed] [Google Scholar]
- 22.Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010;30(18):6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. 30/18/6398[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin KJ, Deng Z, Hamblin M, Zhang J, Chen YE. Vascular PPAR{delta} Protects Against Stroke-Induced Brain Injury. Arterioscler Thromb Vasc Biol. 2011;31(3):574–581. doi: 10.1161/ATVBAHA.110.221267. ATVBAHA.110.221267[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10(2):290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Shi Y, Zhang L, Zhang F, Hu X, Zhang W, Leak RK, Gao Y, Chen L, Chen J. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103. doi: 10.1016/j.nbd.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Wang H, Zhang H, Leak RK, Shi Y, Hu X, Gao Y, Chen J. Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke. Experimental neurology. 2015;272:170–180. doi: 10.1016/j.expneurol.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leak RK, Zhang L, Stetler RA, Weng Z, Li P, Atkins GB, Gao Y, Chen J. HSP27 protects the blood-brain barrier against ischemia-induced loss of integrity. CNS & neurological disorders drug targets. 2013;12(3):325–337. doi: 10.2174/1871527311312030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, Xu N, Stetler RA, Zhang F, Liu X, Leak RK, Keep RF, Ji X, Chen J. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nature communications. 2016;7:10523. doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Yuan L, Zhang X, Hamblin MH, Zhu T, Meng F, Li Y, Chen YE, Yin KJ. Altered long non-coding RNA transcriptomic profiles in brain microvascular endothelium after cerebral ischemia. Experimental neurology. 2016;277:162–170. doi: 10.1016/j.expneurol.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16(4):605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Ren TJ, Qiang R, Jiang ZL, Wang GH, Sun L, Jiang R, Zhao GW, Han LY. Improvement in regional CBF by L-serine contributes to its neuroprotective effect in rats after focal cerebral ischemia. PLoS One. 2013;8(6):e67044. doi: 10.1371/journal.pone.0067044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Q, Wang G, Namura S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2010;30(1):70–78. doi: 10.1038/jcbfm.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeVries AC, Nelson RJ, Traystman RJ, Hurn PD. Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neuroscience and biobehavioral reviews. 2001;25(4):325–342. doi: 10.1016/s0149-7634(01)00017-3. [DOI] [PubMed] [Google Scholar]
- 35.Balkaya M, Krober JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab. 2013;33(3):330–338. doi: 10.1038/jcbfm.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg GA. Ischemic brain edema. Progress in cardiovascular diseases. 1999;42(3):209–216. doi: 10.1016/s0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- 37.Hoiland RL, Bain AR, Rieger MG, Bailey DM, Ainslie PN. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. American journal of physiology Regulatory, integrative and comparative physiology. 2016;310(5):R398–413. doi: 10.1152/ajpregu.00270.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert review of neurotherapeutics. 2015;15(5):523–531. doi: 10.1586/14737175.2015.1035712. [DOI] [PubMed] [Google Scholar]
- 39.Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KM. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995;56(2):127–134. doi: 10.1016/0165-5728(94)00138-e. [DOI] [PubMed] [Google Scholar]
- 40.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25(7):1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 41.Stanimirovic DB, Wong J, Shapiro A, Durkin JP. Increase in surface expression of ICAM-1, VCAM-1 and E-selectin in human cerebromicrovascular endothelial cells subjected to ischemia-like insults. Acta neurochirurgica Supplement. 1997;70:12–16. doi: 10.1007/978-3-7091-6837-0_4. [DOI] [PubMed] [Google Scholar]
- 42.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19(8):819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Becker KJ. Inflammation and acute stroke. Curr Opin Neurol. 1998;11(1):45–49. doi: 10.1097/00019052-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovascular and brain metabolism reviews. 1994;6(4):341–360. [PubMed] [Google Scholar]
- 45.Castillo J, Rodriguez I. Biochemical changes and inflammatory response as markers for brain ischaemia: molecular markers of diagnostic utility and prognosis in human clinical practice. Cerebrovasc Dis. 2004;17(Suppl 1):7–18. doi: 10.1159/000074791. [DOI] [PubMed] [Google Scholar]
- 46.Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. Journal of the neurological sciences. 1994;122(2):135–139. doi: 10.1016/0022-510x(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 47.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enenstein J, Milbauer L, Domingo E, Wells A, Roney M, Kiley J, Wei P, Hebbel RP. Proinflammatory phenotype with imbalance of KLF2 and RelA: risk of childhood stroke with sickle cell anemia. American journal of hematology. 2010;85(1):18–23. doi: 10.1002/ajh.21558. [DOI] [PubMed] [Google Scholar]
- 49.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thrombosis research. 2000;98(3):73–81. doi: 10.1016/s0049-3848(00)00218-8. S0049-3848(00)00218-8[pii] [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa M, Zhang JH, Nanda A, Granger DN. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci. 2004;9:1339–1347. doi: 10.2741/1330. [DOI] [PubMed] [Google Scholar]
- 51.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200–219. doi: 10.1016/j.nbd.2008.08.005. S0969-9961(08)00192-7[pii] [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28(3):229–244. doi: 10.1385/MN:28:3:229. MN:28:3:229[pii] [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackman K, Kahles T, Lane D, Garcia-Bonilla L, Abe T, Capone C, Hochrainer K, Voss H, Zhou P, Ding A, Anrather J, Iadecola C. Progranulin deficiency promotes post-ischemic blood-brain barrier disruption. J Neurosci. 2013;33(50):19579–19589. doi: 10.1523/JNEUROSCI.4318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35(9):2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 56.Fisher M. Injuries to the vascular endothelium: vascular wall and endothelial dysfunction. Rev Neurol Dis. 2008;5(Suppl 1):S4–11. [PubMed] [Google Scholar]
- 57.Rodriguez-Yanez M, Castellanos M, Blanco M, Mosquera E, Castillo J. Vascular protection in brain ischemia. Cerebrovasc Dis. 2006;21(Suppl 2):21–29. doi: 10.1159/000091700. CED2006021S02021[pii] [DOI] [PubMed] [Google Scholar]
- 58.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100(12):1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. 100/12/1686[pii] [DOI] [PubMed] [Google Scholar]
- 59.Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the Kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25(6):1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. 01.ATV.0000165656.65359.23[pii] [DOI] [PubMed] [Google Scholar]
- 60.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr, Garcia-Cardena G, Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199(10):1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103(7):690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, Jain MK. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30(10):1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. J Biol Chem. Kruppel-like factor 4 regulates endothelial inflammation. 2007;282(18):13769–13779. doi: 10.1074/jbc.M700078200. M700078200[pii] [DOI] [PubMed] [Google Scholar]
- 64.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2001;98(16):8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou G, Hamik A, Nayak L, Tian H, Shi H, Lu Y, Sharma N, Liao X, Hale A, Boerboom L, Feaver RE, Gao H, Desai A, Schmaier A, Gerson SL, Wang Y, Atkins GB, Blackman BR, Simon DI, Jain MK. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest. 2012;122(12):4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cowan CE, Kohler EE, Dugan TA, Mirza MK, Malik AB, Wary KK. Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ Res. 2010;107(8):959–966. doi: 10.1161/CIRCRESAHA.110.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: The specific primers for real time PCR
Supplementary Figure 1: Expression of PPARγ protein level in brains of KLF11 KO mice after focal cerebral ischemia. Western blotting data show PPARγ protein level in lysates from cerebral cortex of KLF11 KO and WT mice after 1h MCAO followed by 24h reperfusion. Compared to KLF11 WT controls, there are no significant changes of PPARγ protein levels in brains of KLF11 KO mice at sham or ischemic condition. Representative data are shown from three separate experiments.