Abstract
The homeobox protein homeobox (HOXA9) is a transcriptional factor that regulates patterning during embryogenesis and controls cell differentiation. HOXA9 dysfunction has been implicated in certain cancers. However, the role of HOXA9 in gastric cancer is poorly understood. The present study investigated HOXA9 and its cofactor PBX homeobox 3 (PBX3) expression in patients with gastric cancer. Paired tissue samples from 24 patients and paraffin embedded tissues of gastric cancer patients (104 males and 24 females) were included. HOXA9 and PBX3 expression levels were determined by reverse transcription quantitative polymerase chain reaction in fresh tissues, and by immunohistochemical staining in paraffin embedded tissues. The association between HOXA9/PBX3 expression and clinicopathological features was established. The results demonstrated that HOXA9 and PBX3 mRNA levels were significantly upregulated (P=0.032 for HOXA9 and P=0.031 for PBX3) in gastric cancer tissue. Immunohistochemical staining revealed that HOXA9 expression was associated with differentiation, lymph node metastasis and tumor-node-metastasis (TNM) stage, and PBX3 expression was associated with lymph node metastasis and TNM stage. Correlation analysis revealed a high coincidental expression of HOXA9 and PBX3 levels in gastric cancer (r=0.391; P<0.001). Survival analysis showed that high expression of HOXA9 or PBX3 was associated with poor survival of gastric cancer, and multivariate analysis using Cox's regression model showed that PBX3 expression was an independent prognostic factor in gastric cancer. There was elevated expression of HOXA9 and PBX3 in gastric cancer patients, and high-level expression of those proteins was associated with poor prognosis of gastric cancer. The present study underlines the significance of HOXA9/PBX3 in the development of gastric cancer.
Keywords: homeobox A9, PBX homeobox 3, gastric cancer, progression, prognosis
Introduction
Gastric cancer (GC) is a major public health issue, and is the second leading cause of cancer-associated mortality worldwide, particularly in East Asia (1). Current treatment modalities for GC include surgery, radiotherapy, chemotherapy and their combinations. New therapies, including molecule-targeted therapy, have been prescribed for gastric cancer due to their marked benefits in reducing disease recurrence and increasing long-term survival (2,3). Tumor invasion and metastasis, which are primary causes for treatment failure or mortality among cancer patients, involve multiple steps. The process involves regulation at the molecular level of adhesive molecules, proteolytic enzymes and cell growth and angiogenesis factors, and its mechanism is not yet fully understood (4). Therefore, searching for tumor-specific biomarkers for invasion and metastasis has become necessary for the treatment of GC.
The homeobox (HOX) proteins are transcription factors with roles in development, including regulating the patterning during embryogenesis and the control of cell differentiation (5,6). In mammals, the HOX genes are organized into clusters named A, B, C and D on four separate chromosomes (7). The HOXA cluster contains 12 genes (11 HOX genes and EVX1) and is located in a 155 kb-long genomic region on chromosome 7p15-7p14.2 (8). HOXA9 is normally expressed during development of the female reproductive tract, and its expression is tightly regulated in the adult tract (9,10).
A previous study revealed that deregulated expression of HOX genes is found in cancers (11). However, another study demonstrated that HOX proteins function in a context-dependent manner (5). HOXA9 was revealed to exert a tumor-suppressive effect in breast cancer, reported by Gilbert et al (12). Uchida et al also demonstrated that HOXA9 acts as a tumor suppressor in oral cancer (13). Furthermore, methylation and loss of expression of HOXA9 was reported in oral cavity (14), breast (12,15,16), lung (17), ovarian (18) and bladder (19) cancers.
In contrast to the tumor suppressor role, several studies have considered the oncogenic role of HOXA9 in human cancer (11,20). Ko et al (20) identified that high expression of HOXA9 is associated with poor overall survival (OS) in ovarian cancer, and HOXA9 could promote ovarian tumor growth in vivo. In addition, a previous study also demonstrated that HOXA9 may act as an oncogene in leukemia (11). Therefore, HOXA9 appears to exert its function by interacting with different types of proteins in a tissue-specific manner. However, the role of HOXA9 in gastric cancer is poorly understood.
PBX3 is a member of the PBX family of three-amino acid loop extension HOX genes. PBX proteins are well known for their interaction with HOX proteins that increases the DNA-binding affinity of HOX proteins, thereby enhancing the transcription of the downstream target genes (11,21). A study by Li et al indicated that HOXA/PBX3 interaction is critical for mixed lineage leukemia-induced leukemia (22). The identification of this HOXA/PBX3 gene signature triggered the present study to investigate whether a synergistic effect exists between HOXA9 and PBX3 in GC. The aim of the present study was to evaluate the clinical significance of HOXA9 and PBX3 in the progression and prognosis of GC, and to explore the potential association between HOXA9 and PBX3 in GC progression.
Materials and methods
Patients and tissue samples
The project was approved by the ethics committee on the use of human subjects of Zhejiang Provincial People's Hospital (ZPPH; Hangzhou, China) and written informed consent was obtained from each patient. A total of 24 fresh specimens from patients with GC were acquired from ZPPH between January 2013 and December 2013, and stored at −80°C prior to use. Surrounding normal gastric mucosa samples were also obtained and studied.
In addition, 128 paraffin-embedded specimens of GC were collected at ZPPH between January 2006 and December 2009. All cases were diagnosed clinically at the Department of Gastrointestinal Surgery, and histopathologically at the Department of Pathology of ZPPH. The patient cohort consisted of 104 males and 24 females (Table I), with a median age of 54 years (range, 17–87 years) at the time of surgery.
Table I.
Association of HOXA9 and PBX3 expression with clinicopathological features of patients with gastric cancer.
| Positive HOXA9 expression | Positive PBX3 expression | ||||||
|---|---|---|---|---|---|---|---|
| Clinicopathological features | Total | Patients, n (%) | χ2 | P-value | Patients, n (%) | χ2 | P-value |
| Gender | 0.060 | 0.807 | 0.777 | 0.378 | |||
| Male | 104 | 72 (69.2) | 73 (70.2) | ||||
| Female | 24 | 16 (66.7) | 19 (79.2) | ||||
| Age range | 0.180 | 0.671 | 0.020 | 0.887 | |||
| <60 years | 83 | 56 (67.5) | 60 (72.3) | ||||
| ≥60 years | 45 | 32 (71.1) | 32 (71.1) | ||||
| Differentiation | 14.896 | 0.001 | 1.890 | 0.420 | |||
| Well | 16 | 8 (50.0) | 10 (62.5) | ||||
| Moderate | 51 | 28 (54.9) | 35 (68.6) | ||||
| Poor | 61 | 52 (85.2) | 47 (77.0) | ||||
| Lymph node metastasis | 6.390 | 0.011 | 17.264 | <0.001 | |||
| Negative | 62 | 36 (58.1) | 34 (54.8) | ||||
| Positive | 66 | 52 (78.8) | 58 (87.9) | ||||
| Distant metastasis | 0.924 | 1.000 | 0.795 | 1.000 | |||
| Negative | 126 | 86 (68.3) | 90 (71.4) | ||||
| Positive | 2 | 2 (100.0) | 2 (100.0) | ||||
| TNM stage | 14.396 | 0.0001 | 29.692 | <0.001 | |||
| I+II | 61 | 32 (52.5) | 30 (49.2) | ||||
| III+IV | 67 | 56 (83.6) | 62 (92.5) | ||||
HOXA9, homeobox A9; PBX3, PBX homeobox 3; TNM, tumor-node-metastasis.
All cases were classified according to the World Health Organization pathological classification of tumors. Among the 128 cases of GC, 16 were well differentiated, 51 were moderately differentiated and 61 were poorly differentiated. There were 62 cases without lymph node metastasis, 66 cases with lymph node metastasis, 2 cases with distant metastasis and 126 cases without distant metastasis. According to TNM stage classification, 61 cases were categorized as stage I+II and 67 cases were categorized as stage III+IV. None of the patients had received any radiotherapy or chemotherapy prior to surgery.
All patients were followed for >5 years, and the survival time was calculated from the date of surgery to the deadline for follow-up, or to the date of mortality.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the fresh specimens using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and RNA concentration was determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.). A total of 2 µg of RNA was reverse transcribed using the SuperScript II reverse transcriptase system (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. The cDNA was then subjected to RT-PCR using specific primers with the SYBR Premix ExTaq kit (Takara Bio, Inc., Otsu, Japan). The forward and reverse primers for HOXA9 (NM_152739) were 5′-GTGATGCCATTTGGGCTTATT-3′ and 5′-GGTTTAGAGCCGCTTTGTGC-3′, respectively. Those for PBX3 (NM_001134778) were 5′-CTGTTTGCCTATCCCTGTT-3′ (forward) and 5′-GCAGCAAGTATCTTCGTCTC-3′ (reverse). GAPDH was used as an internal control using the following primers: Forward, 5′-TGAAGGTCGGAGTCAACGG-3′ and reverse, 5′-CTGGAAGATGGTGATGGGATT-3′. The relative amount of mRNA level to GAPDH was calculated as the average 2−ΔΔCq, where ΔCq=Cq-CqGAPDH (23).
Immunohistochemical staining
Each tissue section was baked at 60°C for 2 h, deparaffinized with xylene and rehydrated in graded alcohol. Antigen retrieval was then performed by autoclaving in 0.01 M citrate buffer (pH 6.0) for 3 min. Subsequently, sections were incubated with 3% (v/v) H2O2 for 10 min to block endogenous peroxidase.
To reduce nonspecific reactions, sections were then incubated with 10% (vol/vol) normal goat serum (Histostain-Plus kit; cat. no. 859043; Invitrogen; Thermo Fisher Scientific, Inc.) for 15 min at room temperature. Subsequently, the slides were incubated overnight at 4°C with rabbit polyclonal antibody against human HOXA9 (dilution, 1:500; cat. no. bs6667R; BIOSS, Beijing, China) or rabbit polyclonal antibody to human PBX3 (dilution, 1:500; cat. no. bs12295R; BIOSS). Subsequent to rinsing with PBS, tissue sections were incubated for 20 min at room temperature with biotin-labeled secondary antibody (Histostain-Plus kit; cat. no. 859043; Invitrogen; Thermo Fisher Scientific, Inc.) followed by horseradish peroxidase-linked goat anti-rabbit antibody (Histostain-Plus kit; cat. no. 859043; Invitrogen; Thermo Fisher Scientific, Inc.) for 20 min at room temperature. Sections were then stained with 3,3-diaminobenzidine (ZSGB-BIO, Beijing, China). Finally, the sections were counterstained with hematoxylin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), dehydrated and mounted with a coverslip. Phosphate buffer was used to replace the primary antibody as a negative control.
Evaluation of immunohistochemical staining
Immuno-histochemical staining showed that HOXA9 and PBX3 positive staining were mainly located in the nucleus and cytoplasm. The degree of immunostaining was reviewed under a light microscope (5 fields were viewed with magnification ×200) by two expert pathologists without knowledge of the clinical data and scored independently. The HOXA9 and PBX3 expression level was based on the intensity of cellular staining and the proportion of stained tumor cells.
Staining intensity was scored according to the following criteria: 0, no staining; 1, weak staining (light yellow); 2, moderate staining (yellow brown); and 3, strong staining (brown). The proportion of stained tumor cells was scored according to the proportion of positively stained tumor cells, as follows: 0, <5% positive tumor cells; 1, 6–25% positive tumor cells; 2, 26–50% positive tumor cells; and 3, >51% positive tumor cells. The staining intensity and proportion immunoreactivity scores were then multiplied to obtain a composite score. The values of the composite score ranged from 0 to 9. For additional evaluation, a staining index score of ≤4 was defined as HOXA9 or PBX3 negative expression, and a staining index score of >5 was regarded as HOXA9 or PBX3 positive expression. In cases of discrepancy, a consensus score was chosen for evaluation.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Differences between HOXA9 and PBX3 mRNA expression levels of cancer and normal tissues were determined using the Mann-Whitney U test. χ2 test or Fisher's exact test was used to evaluate the associations between the expression of HOXA9 or PBX3 and the clinicopathological features of the patients with GC. Univariate survival analysis was performed using the Kaplan-Meier method, accompanying the log-rank test to calculate differences among the curves. Multivariate survival analysis was performed to assess predictors associated with prognosis using Cox proportional hazards regression model. Additionally, association between HOXA9 expression, PBX3 expression and clinicopathological features was estimated using Spearman's rank correlation coefficient. All P-values were two-sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Detection of HOXA9 and PBX3 mRNA expression level
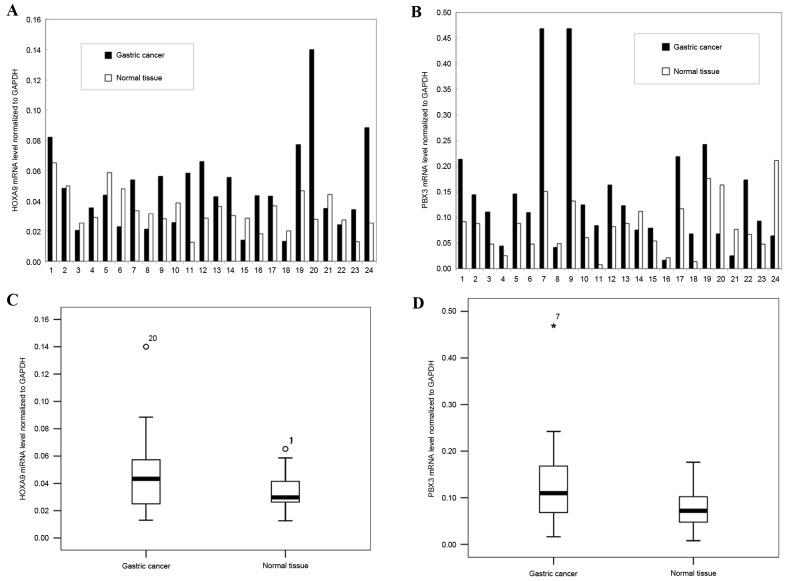
To detect HOXA9 and PBX3 mRNA expression level, a total of 24 paired fresh specimens of GC and surrounding normal mucosa were analyzed using RT-PCR. The results revealed that the HOXA9 mRNA level was upregulated in 62.5% of GCs (15/24), and downregulated in 37.5% of the GCs (9/24), and the mean mRNA level of HOXA9 was upregulated in GC tissues compared with normal tissues (P=0.032; Fig. 1).
Figure 1.
Gene expression of HOXA9 and PBX3 in GC and adjacent normal tissue. (A) The relative mRNA levels of HOXA9 normalized to GAPDH in the 24 paired specimens. (B) The relative mRNA levels of PBX3 normalized to GAPDH in the 24 paired specimens. (C) The mean expression levels of HOXA9 mRNA in GC and in normal tissue (P=0.032). (D) The mean expression levels of PBX3 mRNA in GC and in normal tissue (P=0.031). GC, gastric cancer; HOXA9, homeobox A9; PBX3, PBX homeobox 3. °represents discrete numeric value; *represents extreme values.
Similarly, PBX3 was significantly upregulated in 79.2% of GCs (19/24) and downregulated in the remainder of GCs (5/24, 20.8%), and the mean mRNA level of PBX3 was upregulated in GC tissues compared with normal tissues (P=0.031; Fig. 1).
Further analysis of the association between HOXA9 and PBX3 mRNA level was also performed, and the result showed that the HOXA9 mRNA level was significantly correlated with PBX3 mRNA level (r=0.358; P=0.012; Fig. 2).
Figure 2.
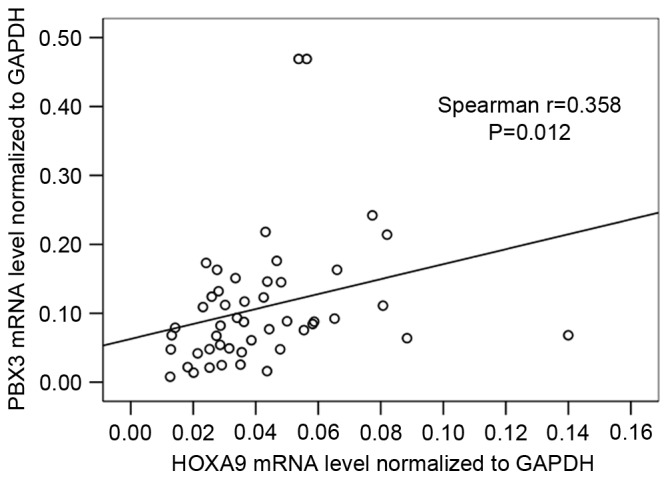
Spearman's rank correlation analysis of HOXA9 and PBX3 mRNA expression. The x-axis shows HOXA9 mRNA level relative to GAPDH, while the y-axis shows PBX3 mRNA level relative to GAPDH. r=0.358; P=0.012. HOXA9, homeobox A9; PBX3, PBX homeobox 3.
Association between HOXA9 and PBX3 expression with clinicopathological features of GC
In order to detect the presence and distribution of HOXA9 and PBX3 expression in GC, immunohistochemical staining was performed, and the association between HOXA9 and PBX3 expression with clinicopathological features of GC was analyzed. The results revealed that immunostaining of HOXA9 was mainly located in the nucleus and cytoplasm of the tumor cells (Fig. 3), and positive expression of HOXA9 was detected in 88 of the 128 patients with GC (68.8%). Additional analysis demonstrated that HOXA9 expression was associated with differentiation, lymph node metastasis and TNM stage (Table I). Gastric cancer patients with poor differentiation, lymph node metastasis and high TNM stage (stages III+IV) had significantly increased expression of HOXA9 compared with those with well or moderate differentiation (P=0.001), no lymph node metastasis (P=0.011) and low TNM stage (stages I+II) (P=0.0001; Table I). The Spearman's rank correlation coefficient of HOXA9 expression with differentiation, lymph node metastasis and TNM stage was 0.185 (P=0.037), 0.298 (P=0.001) and 0.439 (P<0.001), respectively.
Figure 3.
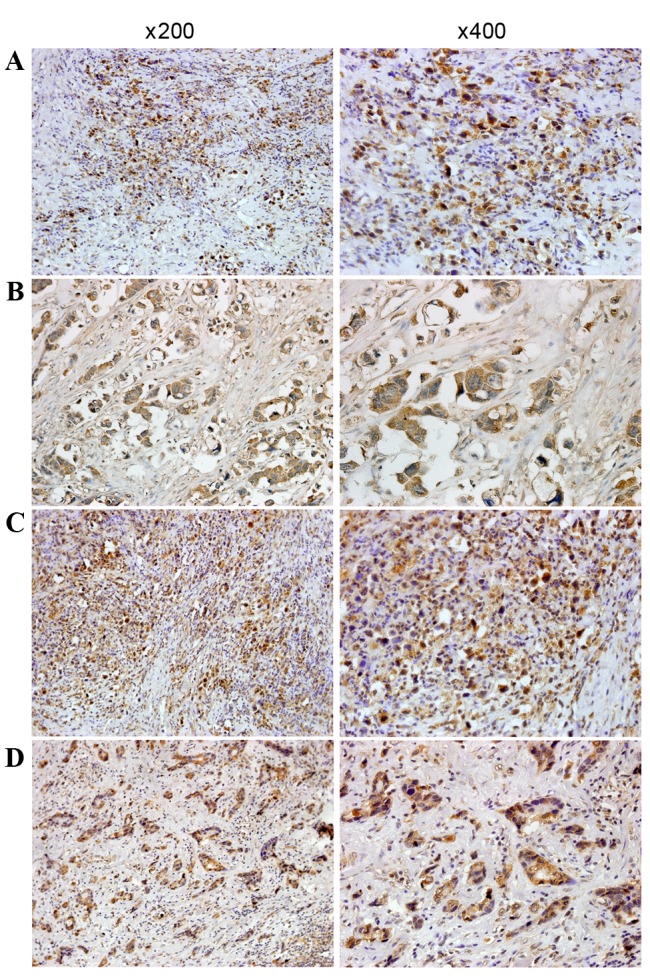
Immunohistochemical staining for HOXA9 and PBX3 in GC and in normal tissue. (A) Immunostaining of HOXA9 in poorly-differentiated GC tissue revealed that positive staining was mainly in the nucleus and also in the cytoplasm. (B) Immunostaining of HOXA9 in moderately-differentiated GC tissue. Positive staining was mainly in the nucleus and cytoplasm. (C) Immunostaining of PBX3 in poorly-differentiated GC tissue. Positive staining was mainly in the nucleus and also in the cytoplasm. (D) Immunostaining of PBX3 in moderately differentiated GC tissue and positive staining was mainly in the nucleus and cytoplasm. GC, gastric cancer; HOXA9, homeobox A9; PBX3, PBX homeobox 3. Magnification, ×200 (left) and ×400 (right).
Immunostaining of PBX3 was predominantly distributed in the nucleus and cytoplasm of the tumor cells (Fig. 3), and positive expression of PBX3 was detected in 92 of the 128 patients with GC (71.9%). PBX3 expression was associated with lymph node metastasis and TNM stage (Table I). Positive expression of PBX3 was detected in 87.9% (58/66) of GC patients with lymph node metastasis, which was increased compared with the expression rate in patients without lymph node metastasis (34/62, 54.8%) (χ2=17.264; P<0.001). The detection rate of PBX3 expression was 92.5% (62/67) in GC patients with TNM stage III+IV, which revealed a significant difference from TNM stage I+II (49.2%; χ2=26.692; P<0.001). Furthermore, the Spearman's rank correlation coefficients of PBX3 expression with lymph node metastasis and TNM stage were 0.438 (P<0.001) and 0.579 (P<0.001), respectively.
Association between expression of HOXA9 and PBX3 in GC
In order to investigate the synergistic effect between HOXA9 and PBX3 in GC, the association between HOXA9 and PBX3 in the development of gastric cancer was analyzed. High coincidental expression of the HOXA9 and PBX3 proteins was observed in gastric cancer. Of the 88 patients that were found to express HOXA9, 69 (78.4%) also expressed PBX3. The correlation between the expression of HOXA9 and PBX3 expression in patients with gastric cancer was statistically significant (r=0.391; P<0.001).
Clinical significance of HOXA9 and PBX3 expression in prognosis of GC
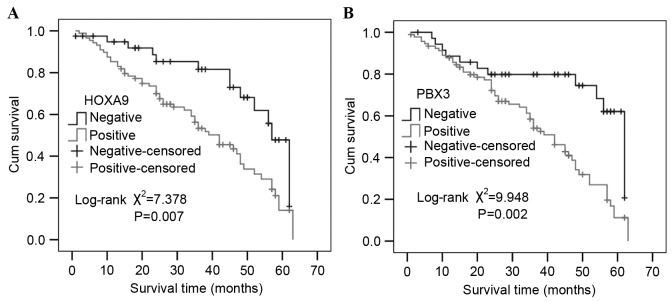
Univariate survival analysis revealed the 3- and 5-year cumulative survival rates were 81.6 and 47.8% in patients with negative HOXA9 expression, and 56.3 and 14.1% in those with positive HOXA9 expression. The mean survival time in patients of GC with positive HOXA9 expression was 37.96±2.22 months, and 50.69±3.02 months for those with negative HOXA9 expression. Evidently, GC patients with positive expression of HOXA9 have a poorer prognosis than those with negative expression (χ2=7.378; P=0.007; Fig. 4).
Figure 4.
Kaplan-Meier survival curve analysis in patients with HOXA9 or PBX3 expression. The cumulative survival rate of patients with positive expression of (A) HOXA9 or (B) PBX3 was significantly decreased compared with the survival rate of patients with negative expression (P=0.007 and P=0.002, respectively). HOXA9, homeobox A9; PBX3, PBX homeobox 3.
Similarly, the 3- and 5-year cumulative survival rates were 79.8 and 62.1% in patients with negative expression of PBX3, which were increased compared with patients with positive expression of PBX3 (58.4 and 11.2%, respectively). The mean survival time in patients of GC with positive expression of PBX3 was 38.13±2.11 months and 50.59±3.46 months for those with negative expression of PBX3. Notably, GC patients with positive expression of PBX3 had a poorer prognosis than those with negative expression (χ2=9.948; P=0.002; Fig. 4). Multivariate analysis using the Cox regression model demonstrated that survival was independently associated with lymph node metastasis (P=0.032) and PBX3 expression (P=0.024; Table II).
Table II.
Multivariate analysis of the correlation between clinicopathological parameters and prognosis in patients with gastric cancer.
| Covariates | Coefficient | Standard error | HR | 95% CI | P-value |
|---|---|---|---|---|---|
| Gender | −0.073 | 0.340 | 0.929 | 0.477–1.810 | 0.829 |
| Age | 0.001 | 0.299 | 1.001 | 0.557–1.799 | 0.998 |
| Differentiation | −0.192 | 0.189 | 0.825 | 0.570–1.196 | 0.310 |
| TNM stage | −1.048 | 0.616 | 0.350 | 0.105–1.171 | 0.089 |
| Lymph node metastasis | 1.166 | 0.542 | 3.209 | 1.109–9.287 | 0.032 |
| Distant metastasis | 0.741 | 0.794 | 2.097 | 0.442–9.942 | 0.351 |
| HOXA9 expression | 0.637 | 0.351 | 1.890 | 0.951–3.758 | 0.069 |
| PBX3 expression | 0.885 | 0.393 | 2.424 | 1.123–5.233 | 0.024 |
HR, hazard ratio; CI, confidence interval; HOXA9, homeobox A9; PBX3, PBX homeobox 3; TNM, tumor-node-metastasis.
Discussion
HOX genes are an important class of patterning regulators that modulate tumor progression and alter tumor cell growth in vitro (24–26). HOX proteins can form heterodimers or heterotrimers with members of the 3-amino-acid loop extension family of cofactors, including PBX and Meis proteins, which may directly regulate the transcription of downstream target genes. Previous studies showed that HOXA9 appears to exert its function by interacting with different types of proteins in a tissue-specific manner (5,11,12,20). However, the role of HOXA9 in gastric cancer has not been fully elucidated.
To understand the clinicopathological significance of HOXA9 in GC, the expression of HOXA9 mRNA level was analyzed in 34 paired fresh GC tissue and 128 paraffin-embedded GC tissues. In the present study, HOXA9 and PBX3 mRNA levels were revealed to be significantly upregulated in GC tissue compared with adjacent normal tissue. Immunohistochemical staining also revealed that gastric cancer patients with poor differentiation, lymph node metastasis and high TNM stage (stages III+IV) had significantly increased expression of HOXA9 compared with patients with well or moderate differentiation (P=0.001), no lymph node metastasis (P=0.011) and low TNM stage (stages I+II; P=0.0001). These results showed that HOXA9 overexpression was involved in the progression of GC. HOXA9 has been implicated in carcinogenesis, since it acts as a transcription factor with roles in development, regulating patterning during embryogenesis and controlling cell differentiation (5,6). Studies also revealed that HOXA9 increases endothelial cell migration and tube formation in human myeloid leukemia cells (27,28). In addition, HOXA9 promotes tumor metastasis by enhancing the adhesion of circulating tumor cells to endothelial cells (29). HOXA9 was also reported to increase cell proliferation and inhibit apoptosis in human glioblastoma (30). These findings indicate that HOXA9 may be involved in tumor progression by modulating interactions between tumor cells and host cells. However, a number of studies revealed that HOXA9 exerted a tumor-suppressive effect in breast cancer, lung cancer, ovarian cancer and bladder cancer (12,17–19). HOXA9 is frequently deregulated in a variety of human cancers, in which it acts as a tumor suppressor or as an oncogene. Although HOXA9 seems to exert its function by interacting with different types of proteins in a tissue-specific manner, the mechanisms underlying these differential functions remain to be identified.
PBX proteins are also well known for their interaction with HOX proteins, which increase the DNA-binding affinity of HOX proteins and thereby enhance the transcription of the downstream target genes (11,31,32). The cooperation between PBX3 proteins and HOXA9 in GC progression is unclear. The present study revealed hat HOXA9 mRNA level was associated with that of PBX3. Immunohistochemical staining also revealed a high coincidental expression of the HOXA9 and PBX3 proteins in GC. Additional analysis revealed hat PBX3 expression was associated with lymph node metastasis and TNM stage. Positive expression rates of PBX3 were increased in GC patients with lymph node metastasis and TNM stage III+IV compared with patients without lymph node metastasis and TNM stage I+II. Therefore, the present data suggest that PBX3 may be a critical cofactor of HOXA9 in GC carcinogenesis and development.
Survival analysis also revealed that high expression of HOXA9 or PBX3 was associated with poor survival of GC, and multivariate analysis using the Cox regression model showed that PBX3 expression was an independent prognostic factor in GC. High HOXA9 expression was reported to be associated with poor OS of epithelial ovarian carcinoma patients (20). Li et al also demonstrated that increased expression of a 4-HOX gene signature (composed of HOXA7, HOXA9, HOXA11 and PBX3) is an independent predictor of shortened OS in patients with cytogenetically abnormal acute myeloid leukemia (33). The present study showed that the HOXA9/PBX3 gene signature has a prognostic value for GC.
On the basis of these findings, it is suggested that PBX3 is a critical cofactor of HOXA9, and that cross-talk between HOXA9 and PBX3 may perform an important role in the mechanism underlying the carcinogenesis, development and progression of GC. Therefore, targeting the interaction of these genes is a feasible strategy for the therapy of GC; however, the mechanisms underlying the regulation of HOXA9/PBX3 in GC development remain to be identified.
Acknowledgements
The present study was supported by the Natural Science Foundation of Zhejiang Province (grant no. LQ16H160017 to Ying-Yu Ma and grant no. LY15H160051 to Xiao-Zhou Mou).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Mullen JT, Ryan DP. Neoadjuvant chemotherapy for gastric cancer: What are we trying to accomplish? Ann Surg Oncol. 2014;21:13–15. doi: 10.1245/s10434-013-3250-9. [DOI] [PubMed] [Google Scholar]
- 3.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO): Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:584–591. doi: 10.1016/j.ejso.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Bogenrieder T, Herlyn M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 5.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 6.Samuel S, Naora H. Homeobox gene expression in cancer: Insights from developmental regulation and deregulation. Eur J Cancer. 2005;41:2428–2437. doi: 10.1016/j.ejca.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 8.Rauch T, Wang Z, Zhang X, Zhong X, Wu X, Lau SK, Kernstine KH, Riggs AD, Pfeifer GP. Homeobox gene methylation in lung cancer studied by genome-wide analysis with a microarray-based methylated CpG island recovery assay. Proc Natl Acad Sci USA. 2007;104:5527–5532. doi: 10.1073/pnas.0701059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng W, Liu J, Yoshida H, Rosen D, Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–537. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]
- 10.Vitiello D, Kodaman PH, Taylor HS. HOX genes in implantation. Semin Reprod Med. 2007;25:431–436. doi: 10.1055/s-2007-991040. [DOI] [PubMed] [Google Scholar]
- 11.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert PM, Mouw JK, Unger MA, Lakins JN, Gbegnon MK, Clemmer VB, Benezra M, Licht JD, Boudreau NJ, Tsai KK, et al. HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J Clin Invest. 2010;120:1535–1550. doi: 10.1172/JCI39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida K, Veeramachaneni R, Huey B, Bhattacharya A, Schmidt BL, Albertson DG. Investigation of HOXA9 promoter methylation as a biomarker to distinguish oral cancer patients at low risk of neck metastasis. BMC Cancer. 2014;14:353. doi: 10.1186/1471-2407-14-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrero-Preston R, Soudry E, Acero J, Orera M, Moreno-López L, Macía-Colón G, Jaffe A, Berdasco M, Ili-Gangas C, Brebi-Mieville P, et al. NID2 and HOXA9 promoter hypermethylation as biomarkers for prevention and early detection in oral cavity squamous cell carcinoma tissues and saliva. Cancer Prev Res (Phila) 2011;4:1061–1072. doi: 10.1158/1940-6207.CAPR-11-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds PA, Sigaroudinia M, Zardo G, Wilson MB, Benton GM, Miller CJ, Hong C, Fridlyand J, Costello JF, Tlsty TD. Tumor suppressor p16INK4A regulates polycomb-mediated DNA hypermethylation in human mammary epithelial cells. J Biol Chem. 2006;281:24790–24802. doi: 10.1074/jbc.M604175200. [DOI] [PubMed] [Google Scholar]
- 16.Sun M, Song CX, Huang H, Frankenberger CA, Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C, Rosner MR. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis. Proc Natl Acad Sci USA. 2013;110:9920–9925. doi: 10.1073/pnas.1305172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son JW, Jeong KJ, Jean WS, Park SY, Jheon S, Cho HM, Park CG, Lee HY, Kang J. Genome-wide combination profiling of DNA copy number and methylation for deciphering biomarkers in non-small cell lung cancer patients. Cancer Lett. 2011;311:29–37. doi: 10.1016/j.canlet.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, Lothe RA, Ahlquist T, Silins I, Tropé CG, Micci F, Nesland JM, Suo Z, Lind GE. DNA methylation profiling of ovarian carcinomas and their in vitro models identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol Cancer. 2007;6:45. doi: 10.1186/1476-4598-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinert T, Borre M, Christiansen A, Hermann GG, Ørntoft TF, Dyrskjot L. Diagnosis of bladder cancer recurrence based on urinary levels of EOMES, HOXA9, POU4F2, TWIST1, VIM and ZNF154 hypermethylation. PLoS One. 2012;7:e46297. doi: 10.1371/journal.pone.0046297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122:3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang CP, Brocchieri L, Shen WF, Largman C, Cleary ML. Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol Cell Biol. 1996;16:1734–1745. doi: 10.1128/MCB.16.4.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Zhang Z, Li Y, Arnovitz S, Chen P, Huang H, Jiang X, Hong GM, Kunjamma RB, Ren H, et al. PBX3 is an important cofactor of HOXA9 in leukemogenesis. Blood. 2013;121:1422–1431. doi: 10.1182/blood-2012-07-442004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Muratovska A, Zhou C, He S, Goodyer P, Eccles MR. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22:7989–7997. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]
- 25.Tan Y, Cheung M, Pei J, Menges CW, Godwin AK, Testa JR. Upregulation of DLX5 promotes ovarian cancer cell proliferation by enhancing IRS-2-AKT signaling. Cancer Res. 2010;70:9197–9206. doi: 10.1158/0008-5472.CAN-10-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinh BQ, Ko SY, Barengo N, Lin SY, Naora H. Dual functions of the homeoprotein DLX4 in modulating responsiveness of tumor cells to topoisomerase II-targeting drugs. Cancer Res. 2013;73:1000–1010. doi: 10.1158/0008-5472.CAN-12-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruhl T, Urbich C, Aicher D, Acker-Palmer A, Zeiher AM, Dimmeler S. Homeobox A9 transcriptionally regulates the EphB4 receptor to modulate endothelial cell migration and tube formation. Circ Res. 2004;94:743–751. doi: 10.1161/01.RES.0000120861.27064.09. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Largaespada DA, Lee MP, Johnson LA, Ohyashiki K, Toyama K, Chen SJ, Willman CL, Chen IM, Feinberg AP, et al. Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nat Genet. 1996;12:154–158. doi: 10.1038/ng0296-154. [DOI] [PubMed] [Google Scholar]
- 29.Bandyopadhyay S, Ashraf MZ, Daher P, Howe PH, DiCorleto PE. HOXA9 participates in the transcriptional activation of E-selectin in endothelial cells. Mol Cell Biol. 2007;27:4207–4216. doi: 10.1128/MCB.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa BM, Smith JS, Chen Y, Chen J, Phillips HS, Aldape KD, Zardo G, Nigro J, James CD, Fridlyand J, et al. Reversing HOXA9 oncogene activation by PI3K inhibition: Epigenetic mechanism and prognostic significance in human glioblastoma. Cancer Res. 2010;70:453–462. doi: 10.1158/0008-5472.CAN-09-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang CP, Shen WF, Rozenfeld S, Lawrence HJ, Largman C, Cleary ML. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995;9:663–674. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- 32.Milech N, Kees UR, Watt PM. Novel alternative PBX3 isoforms in leukemia cells with distinct interaction specificities. Genes Chromosomes Cancer. 2001;32:275–280. doi: 10.1002/gcc.1190. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Huang H, Li Y, Jiang X, Chen P, Arnovitz S, Radmacher MD, Maharry K, Elkahloun A, Yang X, et al. Up-regulation of a HOXA-PBX3 homeobox-gene signature following down-regulation of miR-181 is associated with adverse prognosis in patients with cytogenetically abnormal AML. Blood. 2012;119:2314–2324. doi: 10.1182/blood-2011-10-386235. [DOI] [PMC free article] [PubMed] [Google Scholar]