For refractory or relapsed (r/r) B-cell non-Hodgkin lymphoma (NHL), the response rates to conventional salvage chemotherapy are 27–44%.1 Chimeric antigen receptors (CARs) efficiently redirect T-cell specificity and cytotoxicity to cells expressing the targeted Ag in an HLA-independent manner.2 The early phase clinical trials of CART cells for (r/r) B-cell NHL have demonstrated promising results. Recent updated data from US National Cancer Institute (NCI) showed that five of the seven (71%) evaluable patients with relapsed diffuse large B-cell lymphoma (DLBCL) obtained complete remissions (CRs) after infusion of anti-CD19 CAR-T cells, and four of the five CRs had long-term durability with duration ranging from 38 to 57 months.3 Encouraging results have also been seen in our prior studies of autologous CART-20 cells in patients with r/r CD20+ B-NHL (NCT01735604).4,5 This paper reports the long-term efficacy and safety of CART-20 cells in patients with r/r CD20+ B-NHL after 5-year follow-up.
From July 2012 to June 2015, a total of 17 patients with r/r B-cell NHL have been enrolled in our studies. As of July 2017, the median follow-up time was 20 months (range, 4–60 months). The patients underwent cytoreductive chemotherapy for tumor debulking and lymphodepletion between 3 and 7 days before T-cell infusion. All patients received at least one cycle CAR.20-CD137ζ transduced T cells infusion at a dose of 0.5–1.5×107 kg−1. Clinical trial design and assay protocols have been reported in detail in our prior publications.4,5
The baseline characteristics of all the patients are presented in Table 1. Briefly, all patients were heavily pretreated and had received rituximab previously; 16 patients (94%) had received 4 or more previous treatment regimens, and 12 patients (70.6%) had relapsed after previous second-line chemotherapy regimens. One patient had relapsed post-autologous stem cell transplantation (SCT). Eleven patients (64.7%) were defined as either refractory or progressive according to their responses to recent chemotherapeutics. Fourteen patients (82.4%) were diagnosed with DLBCL and three (17.6%) had indolent lymphoma. Notably, five patients had bulky tumor burden in the phase I trial.
Table 1. Baseline demographic and clinical characteristics.
| Characteristic | All patients (n=17, 100%) |
|---|---|
| Median age, years (range) | 57 (25–85) |
|
Sex, no. (%) | |
| Female | 3 (17.6%) |
| Male | 14 (82.4%) |
|
Disease stage | |
| I, II | 4 (23.5%) |
| III, IV | 13 (76.5%) |
|
IPI group | |
| Intermediate: 2–3 | 15 (88.2%) |
| High: 4–5 | 2 (11.8%) |
| Ki-67: Median (range), % | 75 (60–90%) |
| BM involvement | 3 (17.6%) |
| Median (range) time from diagnosis, years | 3 (0.25–37) |
| Median (range) time from last chemotherapy regimen, months | 4 (1–6) |
| Median (range) number of previous treatment regimens | 7 (2–14) |
|
Type of prior treatment regimen | |
| One or more rituximab treatments | 17 |
| Radiotherapy | 2 |
| AHSCT | 1 |
|
Pathology, no. (%) | |
| Indolent lymphoma | 3 (17.6%) |
| Follicular lymphoma | 1 (5.9%) |
| Mantle cell lymphoma | 1 (5.9%) |
| Primary cutaneous marginal zone lymphoma | 1 (5.9%) |
| Diffuse large B-cell lymphoma | 14 (82.4%) |
| Relapsed after previous therapy with R-CHOP | 1 (5.9%) |
| Relapsed after previous therapy with second-line chemotherapy | 12 (70.6%) |
| Relapsed after previous AHSCT | 1(5.9%) |
|
Tumor burden SPD | |
| SPD>30 | 5 (29.4%) |
| SPD<30 | 12 (70.6%) |
|
Conditioning therapy | |
| None | 4 (23.5%) |
| FC | 1 (5.9%) |
| Others | 12 (70.6%) |
Abbreviations: AHSCT, autologous hematopoietic stem cell transplantation; BM, bone marrow; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; FC, fludarabine and cyclophosphamide; IPI, International Prognostic Index; SPD, the sum of the products of diameters of all index lesions.
Eastern Cooperative Oncology Group (ECOG) scores indicate the performance status of patients with respect to activities of daily living on a scale from 0 to 5, with higher numbers indicating greater disability.
In phase IIa trial, 11 patients were available to evaluate the objective clinical responses. The overall objective response rate was 9 of 11 (81.8%), with 54.5% of patients (6/11) achieving CR and 27.3% (3/11) achieving partial remission (PR). One patient with PR and one patient with stable disease after CART-20 cell infusion received consolidative local radiotherapy and were subsequently converted to CR. In the phase I trial, five of six patients experienced tumor regression.
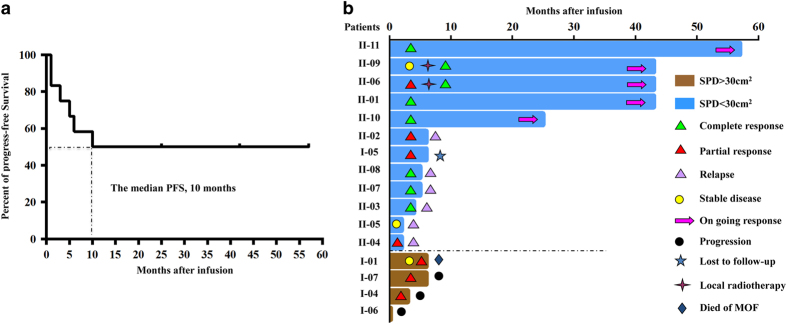
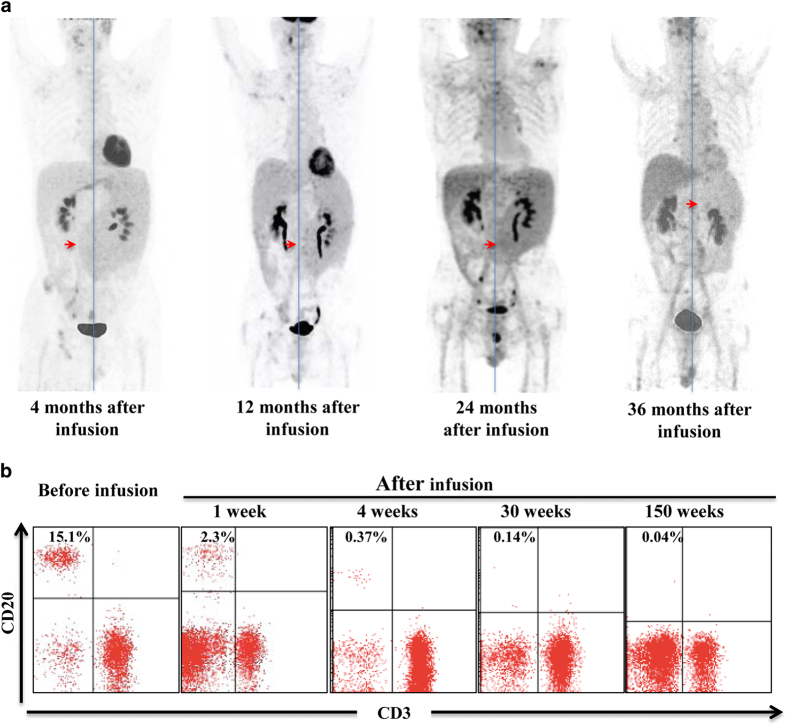
Twelve patients with remission but refusing autologous or allogeneic SCT were followed up for a median of 20 months (range, 4–60 months) from their first CART-20 cell infusion. The estimated median progression-free survival (PFS) of the 12 patients was 10 months (range, 2–57 months) (Figure 1a) and the estimated 2-year rate of PFS was 41.7% (5/12). The longest duration of response (57 months) was seen in UPNII-11 (Figure 1b). The UPNII-09 with advanced refractory marginal zone lymphoma had achieved remission of skin and bone marrow after the first CART-20 cell infusion, but still had enlarged spleen. Thus, UPNII-09 received the consolidated second infusion, and the size of the spleen became gradually smaller and continued to shrink for up to 36 months (Figure 2a). B-cell aplasia of this patient sustained for 150 weeks (Figure 2b and Supplementary Figure).
Figure 1.
Kaplan–Meier curves for PFS and duration responses of all eligible patients. (a) The median PFS was 10 months (95% CI, 2–57 months). (b) Twelve patients with SPD<30 cm2 had clinical improvement; four patients with bulky tumors suffered lymphoma progression.
Figure 2.
The change of the spleen size and CD20+ B-cell counts in the PB of patients UPNII-09. (a) The whole-body 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scans show the larger spleen over the midline of body at 4 months after infusion, then shrink to midline and left midline, respectively, at 12 and 24 months. Especially at 36 months after infusion, the size of spleen become almost normal. (b) The CD20+ lymphocyte cells determined by flow cytometry in PB after CART-20 cell infusion is shown. From 1 week after infusion, the proportion began to decline, and it lasted for over 150 weeks.
The treatment regimen was generally well tolerated. The acute adverse events included temporary chills and fever. The long-term monitoring for adverse events was for 5 years. The delayed adverse events related to CART-20 therapy were summarized in Table 2. No Grade 4 toxicities were observed. Grade 3 herpes zoster due to long-term hypogammaglobulinemia was observed in UPNII-09 at 7 months after infusion. The decrease of immunoglobulin occurred in all patients with B-cell lack. We preventatively administered intravenous immunoglobulin to avoid hypogammaglobulinemia until the B-cell recovery. During the long follow-up periods, no patient was found to have susceptibility to viral infection or increase of other diseases incidence.
Table 2. Adverse events.
| Adverse events |
No. of patients (%, n=16) |
|
|---|---|---|
| All grades | Grade ⩾3 | |
|
Infusion-related events | ||
| Fever | 16 (100.0%) | 0 |
| Rigors | 15 (93.8%) | 1 (6.2%) |
| Fatigue | 3 (18.7%) | 0 |
| Hypotension | 1 (6.2%) | 0 |
| Exudative inflammation of the lungs | 2 (12.5%) | 0 |
| Cytokine release syndrome | 4 (25.0%) | 0 |
| Acute alimentary tract hemorrhage | 2 (12.5%) | 1 (6.2%) |
|
Delayed-events after infusion | ||
| Hematologic events | ||
| Neutropenia | 3 (18.7%) | 1 (6.2%) |
| Thrombocytopenia | 3 (18.7%) | 0 |
| Lymphocytopenia | 11 (68.8%) | 0 |
| Anemia | 2 (12.5%) | 0 |
| Immunology events | ||
| Serum immunoglobulin decrease | 7 (43.8%) | 1 (6.2%) |
| Allergic reaction/hypersensitivity | 1 (6.2%) | 0 |
| Allergic rhinitis | 1 (6.2%) | 0 |
| Autoimmune enteritis | 1 (6.2%) | 0 |
| Vasculitis | 1 (6.2%) | 0 |
| Herpes zoster | 1 (6.2%) | 1 (6.2%) |
| Viral hepatitis | 0 | 0 |
| Other virus infection | 1 (6.2%) | 0 |
| Dermatomycoses | 1 (6.2%) | 0 |
| Bacterial infection | 1 (6.2%) | 0 |
| Nervous system disorder | ||
| Numbness | 1 (6.2%) | 0 |
| Insomnia | 1 (6.2%) | 0 |
| Hypomnesis | 1 (6.2%) | 0 |
| Laboratory abnormalities | ||
| ALT elevation | 2 (12.5%) | 0 |
| AST elevation | 1 (6.2%) | 0 |
| Hyperuricaemia | 2 (12.5%) | 0 |
| Hypoalbuminaemia | 1 (6.2%) | 0 |
| Hypokalemia | 1 (6.2%) | 1 |
| LDH elevation | 2 (12.5%) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotrans- ferase; LDH, lactate dehydrogenase.
Some similar studies of CART cell therapy of B-cell NHL have been reported since 2001.6,7 At the 2016 annual meeting of the American Society of Hematology, updated data from Transcend NHL 001 trial showed that the longest observable PFS of patients with DLBCL was 9 months in those who achieved CR after treatment with CART-19 cells.8 However, in our trials, the longest continued complete remission time of a patient with DLBCL was 57 months, which was comparable to the updated data from NCI group’s trial of CART-19 in DLBCL.3
In conclusion, long-duration CRs were observed in our CART-20 cell therapy. Compared to the outcomes of other clinical trials evaluating CAR-T cells in r/r B-NHL, our CART-20 cell therapy possibly made patients get longer PFS time. Our results provided unique evidence supporting the efficacy and safety of CART-20 in patients with r/r B-cell NHL.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81402566), the grants the Science and Technology Planning Project of Beijing City (No. Z151100003915076 to WDH) and the National Key Research and Development Program of China (No. 2016YFC1303501 and 2016YFC1303504 to WDH).
Footnotes
Supplementary Information accompanies the paper on the Signal Transduction and Targeted Therapy website (http://www.nature.com/sigtrans)
The authors declare no conflict of interest.
References
- Pettengell R, Sebban C, Zinzani PL, Derigs HG, Kravchenko S, Singer JW et al. Monotherapy with pixantrone in histologically confirmed relapsed or refractory aggressive B-cell non-Hodgkin lymphoma: post-hoc analyses from a phase III trial. Br J Haematol 2016; 174: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Guo Y, Han W. Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment. Protein Cell 2017; e-pub ahead of print 2 May 201710.1007/s13238-017-0400-z. [DOI] [PMC free article] [PubMed]
- Kochenderfer JN, Somerville RPT, Lu T, Yang JC, Sherry RM, Feldman SA et al. Long-duration complete remissions of diffuse large B-cell lymphoma after anti-CD19 chimeric antigen receptor therapy. Mol Ther 2017; e-pub ahead of print 21 July 201710.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed]
- Wang Y, Zhang WY, Han QW, Liu Y, Dai HR, Guo YL et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol 2014; 155: 160–175. [DOI] [PubMed] [Google Scholar]
- Zhang W-Y, Wang Y, Guo Y-L, Dai H-R, Yang Q-M, Zhang Y-J et al. Treatment of CD20-directed chimeric antigen receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transd Target Ther 2016; e-pub ahead of print 11 March 201610.1038/sigtrans.2016.2. [DOI] [PMC free article] [PubMed]
- Ghetie MA, Bright H, Vitetta ES. Homodimers but not monomers of Rituxan (chimeric anti-CD20) induce apoptosis in human B-lymphoma cells and synergize with a chemotherapeutic agent and an immunotoxin. Blood 2001; 97: 1392–1398. [DOI] [PubMed] [Google Scholar]
- Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 2012; 119: 3940–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson JS, Palomba L, Gordon LI, Lunning M, Arnason J, Forero-Torres A et al. Transcend NHL 001: Immunotherapy with the CD19-Directed CAR T-Cell Product JCAR017 Results in High Complete Response Rates in Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. Blood 2016; 128: 4192–4192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.