Abstract
BACKGROUND:
Lipomas are the most frequent soft -tissue tumors arising from adipose tissue. Traditionally, open surgery is a mainstay of their treatment. Recently, new treatment modalities emerge in order to decrease morbidity, to increase satisfaction rate in patents, but not to raise recurrence risk at the same time.
AIM:
The aim of this article is to present our experience with liposuction assisted lipoma removal in terms of efficacy, complications, risk of recurrence and patient satisfaction.
METHODS:
The study was prospective in which treated lipomas with vacuum suction were analyzed. Preoperative diagnosis comprised clinical exam and additional diagnostic tools as to rule out malignancy. Subcutaneous lipomas with diameter of at least 5 cm were taken into account. Tumescent liposuction technique with modification was used.
RESULTS:
Lipoma’s size, distribution and demographics are given. Total removal with affordable rate of complication was achieved in each case. No recurrences in 12 months follow-up period were seen. Satisfaction rate in patients was high.
CONCLUSION:
Liposuction assisted lipoma removal is a good alternative to open approach lipectomy and we would recommend its use in selected cases where, it might be more advantageous. However, prospective randomized controlled studies are needed in order to estimate its accurate clinical value.
Keywords: Lipoma, Liposuction, Lipoma removal, Plastic surgery, Suction assisted lipectomy
Introduction
Lipomas are well-defined encapsulated benign tumors arising from adipose tissue comprising the most common mesenchymal tumors in human body [1]. Fat cells are main constituents, but depending on other tissue specimens incorporated, different pathomorphology can be seen. However, the majority of all clinical lipomas are called simple (or conventional) lipomas consisted purely of adipocytes originating from white fatty tissue arranged within fibrovascular stroma [2]. Clinically they are soft circumscripted lesions, occurring everywhere in the body, but mainly subcutaneously on the trunk or extremities [3]. Due to aesthetic disfigurement, discomfort, nerve pressure or cancerophoby, patients seek their removal [4].
Historically, open surgical ablation was the mainstay of their treatment, burdened with scaring and risk of complications. Striving for less scaring, based on accessibility of subcutaneously located simple lipomas, new methods of less invasive treatments have been innovated in last decades [5-9]. Among them, liposuction, as the only FDA approved alternative, receives greatest credits [10]. Soon after its introduction as a tool for aesthetic body contouring, it gained popularity for treatment of other non – cosmetic conditions [11, 12]. Rubenstein et all. were first that used liposuction in lipoma treatment in 1985. [9] The efficacy of liposuction in lipoma removal was arguable at the beginning, with some early reports being positive [12-14] and other not [15].
The aim of this article is to present our first experience with liposuction assisted lipoma removal in terms of efficacy, complications, risk of recurrence and satisfaction in patients with postoperative follow- up of at least 12 months.
Material and Methods
This is a prospective cohort study comprising the patients with lipomas treated with liposuction in the period from September 2013 to July 2016 operated by a same surgeon (the author). All cases were operated at the University Clinic for Plastic and Reconstructive surgery in Skopje where, so far, open extirpation is a standard care treatment for lipoma of any kind. The cohort includes all the patients operated by the author with a minimum follow up of 1 year. Inclusion criteria for utilizing the new method were patients with simple lipoma of the trunk and extremities located above the fascia with diameter at least 5cm in adult patients (18 + years old). Fine - needle aspiration biopsy (FNAB) and ultrasound image with linear probe was conducted in order to exclude any non-lipomatous formations. Occasionally, MRI was utilized in cases where ultrasound was not conclusive. Cases with results stating lipoma were taken into an account (Ist classification group only and clear imaging for lipoma). The new technique was offered to patients with fulfilled aforementioned criteria. After discussing about the advantages and disadvantages of the two methods, the alternative was accepted in studied cases. Patients with positive inclusion criteria that rejected the proposed new method were operated traditionally (open approach) and are not part of this cohort. The same with cases with unclear imaging for lipoma and/or result of FNAB other than Ist classification group. Study has permission from Ethics’ Committee from the Medical Faculty and consent form was signed by all participants. Descriptive statistics are used.
Operative technique: Surgery is conducted in local anesthesia solely or in combination with intravenous sedation by the same surgeon (the author). Around an hour before operation, broad spectrum antibiotic is given i.v. or i.m. as a single shot. Liposuction is done using manually created vacuum with 60ml Toomey syringe after infiltration with modified Klein solution (0.1% lidocaine + 1: 1m adrenaline in 1000ml 0.9% NaCl solution) by means of tumescent “suprawet” technique. It follows classical recommendations for liposuction regarding infiltration, waiting period, suction and respective end points. A ǿ 1mm blunt infiltration cannula and blunt Mercedes ǿ 3mm/ǿ 5mm cannula (Byron®) are used in addition to standard operative tray. Lipoma margins are marked prior infiltration and an entry port as incision is chosen, usually at the most cosmetically acceptable place about 2-5 cm away from margin. Local anesthesia is given at the port (about 2 ml of 1% lidocaine + 0.01% adrenaline). After finishing liposuction, through the same port, all the remaining hard tissue from the cavity are pulled out employing long forceps or paean. Two tissue samples are sent for pathohistological analysis: the solid tissue from the aspirate (after decantation and filtration on gauze) and the residual tissue that is taken away with the forceps at the end. All samples are examined by the same senior pathologist at the Institute for Pathology in Skopje (co-author).
The incision is sutured and a compressive dressing follows. Few hours later, the patient is discharged with an advice for a moderate reduction in daily activities and using analgetics if needed. First check-up is on the third postoperative day. Next check-ups are scheduled depending on further requirements. Wearing a compressive garment or bandage for 3 weeks is advisable. In this period, up to one month, early postoperative complications are evaluated as well as the wound/scar. In the late follow-up period of up to one year and more, the quality of the scar and the liposuctioned surface is assessed. More after, in this period, special attention is paid to any eventual recurrences. If any, this should be further investigated and re-operated by means of traditional open excision. Finally, overall patient satisfaction of the treatment is questioned at the end of 12th month or later, using 1 to 5 rating semi quantitative scale questionnaires. The question is: How you will rate the overall satisfaction of the surgery? (Give marks from 1 to 5, 1 being the worst)
Results
In total, 23 lipomas were operated with this technique. In one patent (case Z.A), there were two lipomas that were treated in the same act. Out of 22 patients, 15 were females (68%), 7 males (32%), aged from 32 to 74 (average age 56). In all cases, lipomas were diagnosed according to proposed pathway: clinically, imaging techniques and FNAB. MRI was employed only in one case where ultrasound couldn’t exclude intramuscular lipoma propagation. Sizes and localizations of lipomas are given in the Table 1. The largest lipoma had dimension 14x26cm. Average size is 9x12cm (first number reflects the smallest dimension and second the largest). The most common localization is back dorsal region (30%). Five lipomas were located on extremities (22%), 17 on trunk (74%) and 1 in posterior neck region.
Table 1.
Demographics, sizes and locations of lipomas
| Patient | Sex | Year of birth | Dimension | Localisation |
|---|---|---|---|---|
| M.M | F | 1967 | 7×10 cm | omaris l. dex. |
| L.J. | M | 1955 | 10×10 cm | nuchae |
| A.J | F | 1982 | 5×13 cm | thoracis lateralis sin. |
| C.K. | F | 1972 | 7×9 cm | dorsi |
| I.G. | M | 1973 | 13×20 cm | parietis abdominis ant.l.sin. |
| T.T. | M | 1953 | 14×26 cm | thoracis lateralis dex. |
| S.Z. | F | 1965 | 7×7 cm | omaris l. sin |
| Z.A. | M | 1959 | 7×7 cm, 8×9 cm | parietis abdominis ant. et thoracis ant. |
| S.A. | M | 1958 | 8×10 cm | femoris dex |
| V.V. | F | 1980 | 8×10 cm | scapularis sin. |
| M.Z. | F | 1945 | 10×14 cm | omaris l.sin |
| N.D. | F | 1943 | 6×8 cm | scapularis dex. |
| S.J. | F | 1949 | 9×11 cm | dorsi proximalis |
| J.N. | M | 1947 | 10×13 cm | omaris l.dex. |
| V.M. | F | 1962 | 6×9 cm | dorsi |
| Lj.D. | F | 1955 | 10×10 cm | dorsi |
| B.N. | F | 1962 | 8×12 cm | dorsi |
| F.B. | F | 1952 | 9×12 cm | dorsi proximalis |
| M.S. | F | 1942 | 13×17 cm | brachii l.dex. |
| M.A. | F | 1954 | 14×20 cm | lumbalis l.sin. |
| O.S. | M | 1963 | 9×10 cm | dorsi |
| C.S. | F | 1955 | 5×7 cm | parietis abdominis anterior |
In early postoperative period, edema and ecchymosis was usual finding that resolved by itself uneventfully. In two cases (8.7%, cases T.T, I.G), there was need of aspiration helping to resolve seroma formation. Obviously these were large lipomas. There was no hematomas, infections, nor problems with wound healing. Pain was light to moderate not affecting daily activities. Wearing the compressive dressing/garments for at least three weeks was unpleasant for some patents, especially when nuchal lipomas were treated. In all cases total lipoma removal was achieved.
Ongoing follow up period is beyond 12 (from 12 to 46 months); 25 months being an average. All cases are still subject of further checkups. In this late postoperative period, there was 0 recurrences so far. Scar diminished by time and it was almost unnoticeable. In three cases, with large lipoma again, (13%, cases I.G., J.N, M.S) skin irregularities were present. Indentations were seen in two cases (8.7%, cases M.S., M.Z). None of that was a concern by a patient. Satisfaction rate at 12th month was high, with an average rate of 4.8. In all cases, the result of the patohistological analysis of the both specimens (aspirate and residual tissue) was an ordinary, conventional lipoma.
Results are summarized in Table 2.
Table 2.
Summarized results
| Patient | Follow up period (months) | Early complication | Late complication | Satisafction mark | |
|---|---|---|---|---|---|
| M.M | 46 | / | / | 5 | Zero reccurence |
| L.J. | 44 | / | / | 5 | |
| A.J | 42 | / | / | 5 | |
| C.K. | 40 | / | / | 5 | |
| I.G. | 40 | Seroma | skin irregularity | 4 | |
| T.T. | 37 | Seroma | / | 5 | |
| S.Z. | 32 | / | / | 5 | |
| Z.A. | 32 | / | / | 5 | |
| S.A. | 27 | / | / | 5 | |
| V.V. | 25 | / | / | 5 | |
| M.Z. | 21 | / | indentation | 5 | |
| N.D. | 20 | / | / | 4 | |
| S.J. | 19 | / | / | 5 | |
| J.N. | 18 | / | skin irregularity | 4 | |
| V.M. | 18 | / | / | ||
| Lj.D. | 18 | / | / | 5 | |
| B.N. | 16 | / | / | 5 | |
| F.B. | 15 | / | / | 5 | |
| M.S. | 15 | / | skin irregularity indentation | 3 | |
| M.A. | 14 | / | / | 5 | |
| O.S. | 13 | / | / | 5 | |
| C.S. | 12 | / | / | 5 |
Photo documentation of two cases is shown in Figure 1 and 2.
Figure 1.

Preoperative photo documentation (1), intraoperative, liposuction of the lipoma (2), early postoperative finding (3) and late postoperative result in the same patient (4)
Figure 2.
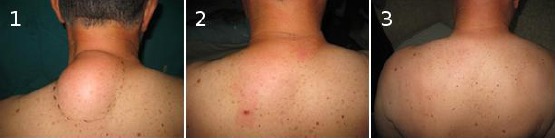
Preoperative (1), early postoperative (2) and late postoperative findings (3) at the same patient with lipoma on the upper dorsal region treated with liposuction
Disscusion
Liposuction is widely accepted method for treatment of obesity, body-contouring and lipomatous disorders [16]. Since its introduction in Europe in mid-1970s by brothers Fishers in Rome and Illouz and Fournier in Paris, with further introduction of Klein’s tumescent technique, it raised high safety and effectiveness rates [17]. So far, lipoma is the most frequent indication of its non – cosmetic application with emerging literature reports [9, 13-15, 18-25]. Advantages are smaller scars, less pain, good cost – effectiveness, shorter operative time, lower complication rates, better final surface contour, high patient compliance and satisfaction, ability to remove more lipomas through fewer incisions, ability to remove a tumor from distant operative site aesthetically acceptable [12].
On the other hand, it is highly safe procedure. [26] In our opinion, great indication for liposuction is suprafascial lipomatous masses, uni- or multilateral with moderate to large size where diagnosis is well established. However, there are reports for removing smaller lipomas in areas where scar is to be avoided [19]. Our cohort includes cases of subcutaneous lipoma with 5+ cm diameter where utilizing liposuction seems reasonable. Unless remote scar is wanted, we think that smaller lipomas can be easily removed via small incisions and squeezing technique thus unnecessarily employing liposuction.
Several drawbacks have an impact on broader use of liposuction in lipoma treatment world widely. The need for special instrumentation is one of them. Others are tissue fragmentation and possible higher recurrence risk due to the closed approach [19]
The main concern with tissue fragmentation deals with accurate pathology analysis of the sample that has much with the fear of malignances. Several studies have demonstrated that cell integrity in lipoaspirate is not damaged thus adequate pathohistology can be done accordingly [27, 28]. Moreover, in order to exclude malignancy, preoperative diagnosis of lipoma is mandatory and sufficient data must be collected prior any liposuction. One should always bear in mind that atypical lipomas or liposarcoma might have similar appearances [29]. Therefore, fine needle aspiration biopsy and ultrasound imaging should be the minimal supplement [18]. In doubtful cases, MRI should be added, especially when tumor growth is sudden or painful, size larger than 10 cm, localization atypical. It is a highly sensitive and specific imaging technique for soft tissue tumors [29, 30]. Furthermore it determines the localization and eventual intramuscular propagation. In highly suspicious cases, open biopsy should be done finally, in order to exclude liposarcoma of any type [29]. Liposarcoma treatment differs and its liposuction is extremely unpleasant scenario [31]. Having in mind the above, with accurate preoperative diagnosis and further validation with lipoaspirate pathohistology, misdiagnosis can be annulated. We have used ultrasound with linear probe in all cases as well as FNAB prior treatment. Only in one case, MRI had to be done in order to exclude intramuscular propagation. The pathologist had no difficulties with tissue samples and all results showed simple lipomas and their prospective capsule.
The higher possible recurrence risk in lipoma treated with liposuction compared to traditional removal is a conclusion deducted by observation or small studies. As a closed method of removal with limited visualization, this statement might be true for liposuction due to contingent incomplete removal of lipomatous or capsular/hard residual tissue [25]. However, it seems that the deduction is premature. Recurrence risk in open lipectomy is around 2% [29]. All studies published in the literature about suction assisted lipectomy have limited number of cases that can estimate that risk of 2% or less [25]. Up to date, there is only one small comparative study with 30 cases included, reporting unacceptable higher recurrence risk in liposuction treated cases [15]. Still, this study included giant lipomas only and is one of the oldest that might have implication on technical skills at that time. Apesos reported no recurrence in 4 patients with moderate and large lipoma treated with suction within a follow up period of 3 years [20]. In the prospective study of Wilhelmi et all., no recurrence have been seen in a follow - up of 1-10 years in 5 cases [19]. Case reports for suction- treated giant lipomas showed no recurrence in a follow up of 2 years [22, 23]. Recent studies advocate the effectiveness. Al-Basty and El-Khatib [21], after the liposuction advice capsule extirpation with forceps through the same incision or through counter incision if lipoma is larger. Adding this modification, no recurrence has been seen in 16 patients in a long term follow- up of 6 years. Choi et all., used the same modification and had zero recurrence in 12 patients followed-up for a period of 2 years [18]. The largest and most recent published study has also applied the proposed modification and reports no recurrence in 44 treated lipomas (in 23 patients) for a period of mean follow up of 6 years [25]. Extraction of the capsule/hard residual tissue looks reasonable as it might have lipomatous precursors that can lead to future lipoma recurrence if untreated [32]. Adding the modification means radical ablation which decreases or annuls recurrence risk. We had used the modification in most of the cases. Our observations show that in some lipomas, especially ones with shorter history or location other than dorsal region, the hard residual tissue/capsule can be suctioned or mechanically destroyed.
There is no study in the literature about complication of suction treated lipoma, thus we cannot compare our results. If liposuction as a technique is taken into account, then we can say that the rates are acceptable [33]. All the complications are minor (local) and no major (systemic) complications were advocated. There was no need of further treatments. However, the study has limitations concerning small number of participants and its uncontrolled design.
In conclusion we can say that in properly selected cases of lipoma, that are sufficiently preoperatively examined with adequate operative technique used, liposuction - assisted lipectomy may have an advantage over the classical open technique, which vast majority, still, assumes it as golden standard. It counts especially in patients where larger scar is an issue. Nevertheless, larger randomized prospective studies are needed to evaluate precisely its clinical value.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Plötz S, et al. Häufige Hauttumoren in der Praxis. Berlin Heidelberg: Springer-Verlag; 2012. p. 38. https://doi.org/10.1007/978-3-642-24702-6. [Google Scholar]
- 2.Shiffman AM. Lipomatous Disorders:Classification and Review of the Literature. J Cosm Surg Med. 2005;5(3):26–36. [Google Scholar]
- 3.Koh HK, Bhawan J. Tumors of the skin. In: Moschella SL, Hurley HJ, editors. Dermatology. 3d ed. Philadelphia: Saunders; 1992. pp. 1721–1808. [Google Scholar]
- 4.Hodl S. Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC, Landthaler M, editors. Regionale und spezielle Erkrankungen des Fettgewebes. Dermatologie und Venerologie. 2005;5:1021–23. PMCid:PMC548971. [Google Scholar]
- 5.Goldman A, Wollina U. Lipoma treatment with a subdermal Nd:YAG laser technique. Int J Dermatol. 2009;48(11):1228–32. doi: 10.1111/j.1365-4632.2008.04007.x. https://doi.org/10.1111/j.1365-4632.2008.04007.x PMid:20064183. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Jung JY, Roh MR, Chung KY. Treatment of Lipomas using a Subdermal 1,444-nm Micropulsed Neodymiumdoped Yttrium Aluminum Garnet Laser. Dermatolog Surg. 2011;37(9):1375–1376. doi: 10.1111/j.1524-4725.2011.02084.x. https://doi.org/10.1111/j.1524-4725.2011.02084.x PMid:22988996. [DOI] [PubMed] [Google Scholar]
- 7.Rotunda AM, Ablon G, Kolodney MS. Lipomas Treated with Subcutaneous Deoxycholate Injections. J Am Acad Dermatol. 2005;53:973–978. doi: 10.1016/j.jaad.2005.07.068. https://doi.org/10.1016/j.jaad.2005.07.068 PMid:16310057. [DOI] [PubMed] [Google Scholar]
- 8.Hallock GG. Endoscope-Assisted Suction Extraction of Lipomas. Ann Plast Surg. 1995;34(1):32–34. doi: 10.1097/00000637-199501000-00007. https://doi.org/10.1097/00000637-199501000-00007 PMid:7702298. [DOI] [PubMed] [Google Scholar]
- 9.Rubenstein R, Roenigk H, Garden JM, Goldberg NS, Pinski JB. Liposuction for lipomas. J Dermatol Surg Oncol. 1985;11(11):1070–1074. doi: 10.1111/j.1524-4725.1985.tb01395.x. https://doi.org/10.1111/j.1524-4725.1985.tb01395.x PMid:4056191. [DOI] [PubMed] [Google Scholar]
- 10.Boyer M, et al. A review of techniques and procedures for lipoma treatment. Clinic Dermatol. 2015;3(4):105–112. https://doi.org/10.11138/cderm/2015.3.4.105. [Google Scholar]
- 11.El-Khatib HA. Liposuction for chronic medical diseases and noncosmetic conditions:review of the literature. Plast Aesthet Res. 2015;2:1–6. https://doi.org/10.4103/2347-9264.149362. [Google Scholar]
- 12.Coleman WP., 3rd Noncosmetic applications of liposuction. J Dermatol Surg Oncol. 1988;14(10):1085–1090. doi: 10.1111/j.1524-4725.1988.tb03465.x. https://doi.org/10.1111/j.1524-4725.1988.tb03465.x. [DOI] [PubMed] [Google Scholar]
- 13.Spinowitz AL. Liposuction surgery:an effective alternative for treatment of lipomas. Plast Reconst Surg. 1990;86(3):606–609. https://doi.org/10.1097/00006534-199009000-00059 PMid:2385686. [PubMed] [Google Scholar]
- 14.Pinski KS, Roenigk HH., Jr Liposuction of lipomas. Dermatol Clin. 1990;8(3):483–92. PMid:2199109. [PubMed] [Google Scholar]
- 15.Raemdonck D, De Mey A, Goldsehmidt D. The treatment of giant lipomas. Acta Chir Belg. 1992;92:213. PMid:1414141. [PubMed] [Google Scholar]
- 16.Kryger ZB. Liposuction. In: Kryger ZB, Sisco M, editors. Practical Plastic Surgery. Austin, TX: Landes Bioscience; 2009. p. 430. [Google Scholar]
- 17.Flynn TC. The history of liposuction. In: Shiffman MA, Di Giuseppe A, editors. Liposuction:Principles and Practice. New York: Springer-Verlag Berlin Heidelberg; 2006. pp. 3–6. https://doi.org/10.1007/3-540-28043-X_1. [Google Scholar]
- 18.Choi CW, Kim BJ, Moon SE, et al. Treatment of lipomas assisted with tumescent liposuction. J Eur Acad Dermatol Venereol. 2007;21:243–246. doi: 10.1111/j.1468-3083.2006.02037.x. https://doi.org/10.1111/j.1468-3083.2006.02037.x PMid:17243961. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelmi BJ, Blackwell SJ, Mancoll JS, Phillips LG. Another indication for liposuction:small facial lipomas. Plast Reconstr Surg. 1999;103(7):1864–7. doi: 10.1097/00006534-199906000-00008. https://doi.org/10.1097/00006534-199906000-00008 PMid:10359246. [DOI] [PubMed] [Google Scholar]
- 20.Apesos J, Chami R. Functional applications of suction-assisted lipectomy:A new treatment for old disorders. Aest Plast Surg. 1991;15(1):73–79. doi: 10.1007/BF02273837. https://doi.org/10.1007/BF02273837. [DOI] [PubMed] [Google Scholar]
- 21.Al-basti HA, El-Khatib HA. The use of suction-assisted surgical extraction of moderate and large lipomas:long-term follow-up. Aest Plast Surg. 2002;26(2):114–7. doi: 10.1007/s00266-002-1492-1. https://doi.org/10.1007/s00266-002-1492-1 PMid:12016495. [DOI] [PubMed] [Google Scholar]
- 22.Ilhan H, Tokar B. Liposuction of a pediatric giant superficial lipoma. J Pediatr Surg. 2002;37(5):796–8. doi: 10.1053/jpsu.2002.32291. https://doi.org/10.1053/jpsu.2002.32291 PMid:11987105. [DOI] [PubMed] [Google Scholar]
- 23.Nichter LS, Gupta BR. Liposuction of giant lipoma. Ann Plast Surg. 1990;24:362. doi: 10.1097/00000637-199004000-00011. https://doi.org/10.1097/00000637-199004000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Calhoun KH, Bradfield JJ, Thompson C. Liposuction assisted excision of cervicofacial lipomas. Otolaryngol Head Neck Surg. 1995;113(4):401–403. doi: 10.1016/S0194-59989570075-7. https://doi.org/10.1016/S0194-5998(95)70075-7. [DOI] [PubMed] [Google Scholar]
- 25.Copeland-Halperin LR, Pimpinella V, Copeland M. Combined liposuction and excision of lipomas:long-term evaluation of a large sample of patients. Plastic Surgery International. 2015;2015 doi: 10.1155/2015/625396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Housman TS, Lawrence N, Mellen BG, et al. The safety of liposuction:results of a national survey. Dermatol Surg. 2002;28:971–978. doi: 10.1046/j.1524-4725.2002.02081.x. https://doi.org/10.1097/00042728-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Campbell GLM, Laudenslager N, Newman J. The effect of mechanical stress on adipocyte morphology and metabolism. Am J Cosm Surg. 1987;4:89–94. https://doi.org/10.1177/074880688700400202. [Google Scholar]
- 28.Shiffman MA, Mirrafati S. Fat transfer techniques:the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg. 2001;27(9):819–826. doi: 10.1046/j.1524-4725.2001.01062.x. https://doi.org/10.1097/00042728-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Dalal KM, Antonescu CR, Singer S. Diagnosis and management of lipomatous tumors. J Surg Oncol. 2008;97:298–313. doi: 10.1002/jso.20975. https://doi.org/10.1002/jso.20975 PMid:18286473. [DOI] [PubMed] [Google Scholar]
- 30.Gaskin CM, Helms CA. Lipomas, Lipoma Variants, and Well-Differentiated Liposarcomas (Atypical Lipomas):Results of MRI Evaluations of 126 Consecutive Fatty Masses. AJR. 2004;182:733–739. doi: 10.2214/ajr.182.3.1820733. https://doi.org/10.2214/ajr.182.3.1820733 PMid:14975977. [DOI] [PubMed] [Google Scholar]
- 31.Voulliaume D, Vasseur C, Delaporte T, Delay E. An unusual risk of liposuction:liposuction of a malignant tumor. About 2 patients [Article in French] Ann Chir Plast Esthet. 2003;48(3):187–193. doi: 10.1016/s0294-1260(03)00043-8. https://doi.org/10.1016/S0294-1260(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 32.Suga H, Eto H, Inoue K, et al. Cellular and molecular features of lipoma tissue:comparison with normal adipose tissue. Br J Dermatol. 2009;161:819–825. doi: 10.1111/j.1365-2133.2009.09272.x. https://doi.org/10.1111/j.1365-2133.2009.09272.x PMid:19558598. [DOI] [PubMed] [Google Scholar]
- 33.Dixit VV, Wagh MS. Unfavorable outcomes of liposuction and their treatment. Indian J Plast Surg. 2013;46(2):377–792. doi: 10.4103/0970-0358.118617. https://doi.org/10.4103/0970-0358.118617 PMid:24501474 PMCid:PMC3901919. [DOI] [PMC free article] [PubMed] [Google Scholar]


