Abstract
Tissue chips are poised to deliver a paradigm shift in drug discovery. By emulating human physiology, these chips have the potential to increase the predictive power of preclinical modeling, which in turn will move the pharmaceutical industry closer to its aspiration of clinically relevant and ultimately animal-free drug discovery. Despite the tremendous science and innovation invested in these tissue chips, significant challenges remain to be addressed to enable their routine adoption into the industrial laboratory. This article describes the main steps that need to be taken and highlights key considerations in order to transform tissue chip technology from the hands of the innovators into those of the industrial scientists. Written by scientists from 13 pharmaceutical companies and partners at the National Institutes of Health, this article uniquely captures a consensus view on the progression strategy to facilitate and accelerate the adoption of this valuable technology. It concludes that success will be delivered by a partnership approach as well as a deep understanding of the context within which these chips will actually be used.
Impact statement
The rapid pace of scientific innovation in the tissue chip (TC) field requires a cohesive partnership between innovators and end users. Near term uptake of these human-relevant platforms will fill gaps in current capabilities for assessing important properties of disposition, efficacy and safety liabilities. Similarly, these platforms could support mechanistic studies which aim to resolve challenges later in development (e.g. assessing the human relevance of a liability identified in animal studies). Building confidence that novel capabilities of TCs can address real world challenges while they themselves are being developed will accelerate their application in the discovery and development of innovative medicines. This article outlines a strategic roadmap to unite innovators and end users thus making implementation smooth and rapid. With the collective contributions from multiple international pharmaceutical companies and partners at National Institutes of Health, this article should serve as an invaluable resource to the multi-disciplinary field of TC development.
Keywords: Tissue chips, microphysiological systems, toxicology, pharmacokinetics, innovation, partnership
Introduction
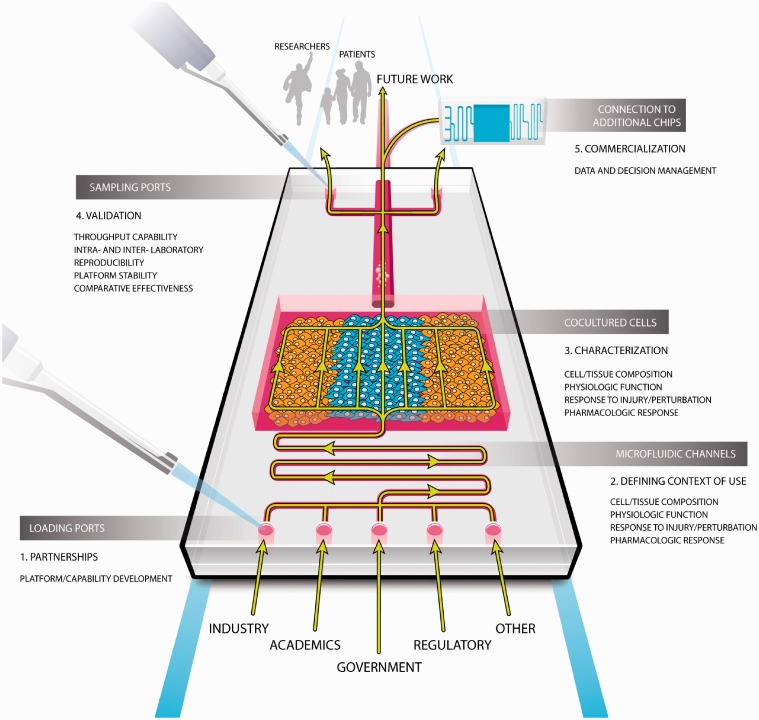
Quality of life and life expectancy have increased in part due to the discovery and market approval of innovative medicines. Such medicines are increasingly moving beyond symptomatic control and towards modifying disease progression in a patient-centric and disease-specific manner. But, the current drug discovery process is vulnerable to poor prediction of human physiological responses especially relating to age-, sex-, and patient-specific phenomena;1 so ensuring clinical translation will be central to accelerating the next generation of innovative medicines. We believe that TCs will enable a paradigm shift in drug discovery by increasing the predictive power of early preclinical modeling with their distinctive bioengineered features more accurately emulating human physiology.2,3 In the United States, TC development has received considerable support from the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS) and Defense Advanced Research Program Agency (DARPA) in partnership with the US Food and Drug Administration (FDA). NCATS has also engaged the partnership of the Innovation and Quality Consortium’s (IQ) Microphysiological Systems Working Group. These pharmaceutical scientists from 13 different companies are uniquely positioned to provide insights into uses and performance expectations for novel technologies that might have use in drug development given their deep engagement and experience in contemporary approaches. In this article, the IQ working group has partnered with NCATS colleagues to present a collaborative view on opportunities for early applications of TCs by sharing in which context their use will generate the best immediate value, build confidence, and advance the field. We also define a path forward or roadmap to the expedited development, characterization, and validation of TCs within drug discovery and make recommendations on the responsible parties at each step. Figure 1 pictorially represents the main themes discussed within this manuscript.
Figure 1.
Pictorial representation of the key aspects of the roadmap that have been discussed within the manuscript. (A color version of this figure is available in the online journal.)
TCs as novel preclinical modeling platforms offer a number of unique opportunities, with improved clinical predictivity the most apparent and the greatest hope for this innovation. But, they may also provide a more efficient approach to mechanistic investigation, early safety liability screening and even more translationally relevant modeling of drug distribution and metabolism. Species-specific chips could provide novel insights into comparative animal and human biology. In the opinion of these authors, TCs are unlikely to completely replace animal studies in the near term but they can certainly complement and reduce their use in certain cases. A future of animal-free drug development is a reasonable aspiration if we are deliberate in our development of relevant alternatives. This manuscript intends to bring that focus to TCs.
Partnership
Innovation often generates considerable early excitement and a lot of uncoordinated efforts to assess the opportunity. In drug development, this approach has not historically facilitated rapid, efficient and widespread adoption and hence impact of novel technologies. Accordingly, we believe that establishing successful partnerships is therefore a crucial first step and a shared responsibility between the TC innovators and the end users such as pharmaceutical scientists. We recommend multi-party frameworks (e.g. consortia) as a good mechanism for TC partnership but stress the need for focus and agility to keep pace with innovation. Partners should establish early alignment on basic concepts and shared goals. They should also recognize the differing incentives and goals across sectors and facilitate mutual success by, for example, ensuring “drugs” provided for characterization and validation are cleared for inclusion in academic publications. NCATS, working with AstraZeneca, GSK, Pfizer, Roche/Genentech and Sanofi, has already established a material transfer agreement (MTA) to expedite and simplify this process. The use of well-vetted template agreements, such as MTA or Memorandum of Understanding (MOU) has greatly facilitated the partnerships between pharmaceutical partners and NCATS and has tremendously decreased the transaction time it takes to come to terms. Through these partnerships, NCATS was able to broker and facilitate the transfer of relevant compounds specific for a particular TC from its pharma partner to TC developer for use characterization and validation. Securing funding is a fierce, competitive process, so by setting out key needs, real life challenges and agreeing on the attributes of each TC, the pharmaceutical scientists aspire to maximize the available funds and the return on the investment by providing a consistent guidance to academics and vendors alike.
Context of use
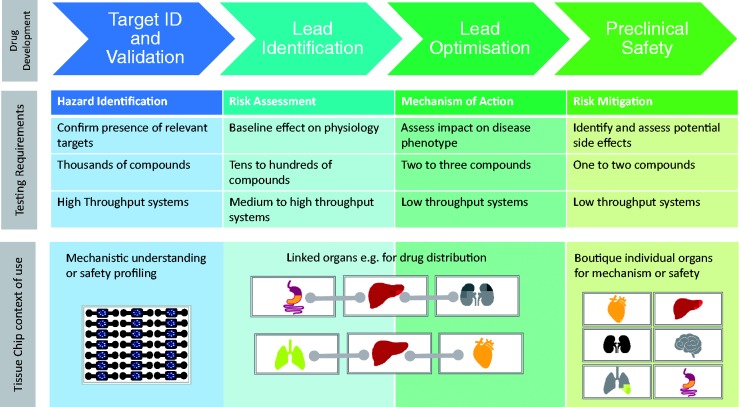
There are a number of scenarios along the drug development progression pathway where TCs might be applied representing discrete “contexts of use.” Each of these contexts has a different set of technical standards or requirements and potential value proposition (Figure 2). These contexts are also associated with a standard against which success would be measured and a threshold of confidence that would need to be achieved to foster uptake and application. These uses are not necessarily mutually exclusive but may also not be interchangeable in that a system built for one application may not be fit for another. Pharmaceutical partners should define use scenarios, some insights into technical standards and approaches to characterization and validation thus facilitating rapid uptake by end users, creation of near term value and confidence building across the field. As the adoption of novel technologies is often an evolutionary process, any initial context of use does not and should not restrict future uses. We believe that there are three primary contexts of use that provide initial value and points of evolutionary traction for the first generation of TCs.
Figure 2.
Illustration of the purpose of the phases of early drug development and how the context of use of a TC will vary depending upon the phase in which it is intended to be used. It is conceivable that boutique assays and/or linked systems can be used in other phases too but at least in the first generation of these devices, the proposed deployment is preferred. (A color version of this figure is available in the online journal.)
Mechanistic investigation
TCs that would support mechanistic investigation of either pharmacology or toxicity are likely to be a popular initial context of use with published examples already available.4 Combination with technologies such as iPSC and CRISPR cas9 offer unique opportunities to test functional consequences of making precise targeted changes to the genome of living cells.5 TCs will enable efficient responses to development-limiting liabilities by facilitating rapid assessment of on- or off-target mechanisms. Such understanding will provide a platform for screening new drug candidates which are safer as the off-target effect once identified can be chemically “designed out” or an accurate “black box” warning to specific patient populations can be included. TCs may also be used to discover new biomarkers of toxicity or disease as well as offer opportunities to increase disease understanding especially when this is not attainable in animal models, something that we will see more often as the pharmaceutical industry learn more from human genome sequencing and editing. This was recently demonstrated with a human gut-on-a-chip micro-device where the authors found that immune cells and lipopolysaccharide together stimulated epithelial cells to produce pro-inflammatory cytokines that induced villus injury leading to compromised intestinal barrier function. The TC on this occasion was able to provide unique insights and as such enhanced the understanding of mechanisms involved in human gut pathophysiology.6
Technical standards for use in mechanistic investigations would largely be defined by the biology or pathobiology of the site of action to be investigated. Accordingly, the focus is more localized and defined than a screening assay might need to be. The number of compounds that are generally considered in an investigative application are generally small (e.g. no more than 10), so analytical throughput would not be a significant limitation. The use would be bespoke and sporadic so large supplies and costs also would not be limiting. Initial applications would likely prioritize or triage very similar molecules for a known parameter with a view to inform internal decision-making and thus would require a lower confidence burden compared to that which would inform regulatory decisions.
Preclinical safety screening
Safety liability screening presents a broader opportunity with a greater potential value proposition. For efficiency, we propose a prioritization of TC development aligned to common target organ sources of safety-related compound attrition. The cardiovascular, hepatic, renal, and gastrointestinal organ systems are frequently cited as those that drive a significant proportion of safety-related attrition.7 Each of these organ systems has unique physiology, multiple cellular targets and manifestations of toxicity and biomarkers of those injuries to consider. In the case of the cardiovascular system, the myocardium, heart valves, and intra- and extra-cardiac arteries are all primary targets of both structural and functional toxicity.8 The ability to recapitulate these toxicities in an in vitro model would offer considerable value. But, given the challenges of recreating the entire system in a single platform, more than one TC may be required. That is likely true for the other organs as well. A portfolio of relevant TCs applied as a pre-animal safety screen offers a significant opportunity to identify development-limiting liabilities earlier in development when mitigation strategies (e.g. drug design) are most available and efficient.
Technical standards for screening assays or platforms are a little more challenging given the diversity of toxicologic mechanisms and cellular targets that may need to be represented. In vivo animal systems engender inherent confidence because they more closely replicate the complexity of integrative physiology than any non-animal system can at this time. For safety assessment, we are most concerned about the liabilities we do not expect that might involve biology we do not yet understand. Accordingly, the hurdle of confidence is greater than for a mechanistic platform. But it would be a higher hurdle to replace an animal model and it could fill a current gap in our development scheme. A screening platform would also need to accommodate a bit greater throughput (e.g. 10s) and warrant greater scrutiny of the economics given the need for a “portfolio” of assays.
Drug disposition
The third use for TCs could be in determination of human drug absorption, distribution, metabolism and excretion (ADME). In this area, TCs will need to demonstrate higher value (e.g. superiority in its predictive capacity and cost or speed advantages) as current methods have reasonable success in predicting human pharmacokinetics (PK). Key PK parameters are clearance, volume of distribution and fraction absorbed (Fa). For clearance, human hepatocytes and microsome preparations are routinely used and have a high level of confidence associated with the resultant data. The same applies for Fa where an analysis of permeability via Caco-2 and solubility is performed. Application of TCs for PK may involve single organ TC platforms, but the value of linked platforms should not be overlooked, especially when considering the volume of distribution which is most reliably predicted from in vivo PK experiments in conjunction with known plasma protein binding. Linked or multi-organ microphysiological systems are an aspirational intent of both the NCATS and DARPA Tissue Chip programs. Multi-organ systems are starting to be described in the literature.9,10 Given the challenges of representing the influences of liver metabolism in single organ or 2D cell culture systems, linking liver to other important target organs of toxicity (e.g. kidney, intestine) would be a useful starting point for exploring the value of linked systems. The current challenge is that many of the today's TCs are partial representations of the complete organ system which will in turn complicate their relevance in a linked system. We therefore encourage investigators to have a clear understanding of the respective individual chip performance, reproducibility and relevance before undertaking significant efforts to link them.
Building the platform
The construct of relevant and useful TCs including their biological content, microfluidic dynamic properties and throughput should be aligned to their intended context of use. Since TCs provide an opportunity to understand comparative species biology, their cellular source may be animal or human. The varying responses of different species and, at times, even strains of individual species are well recognized.11–13 Species selection for translational animal studies for either efficacy or safety are often more traditional than evidence based. Species-specific TCs could provide an ability to gain informed insights into species-specific drug responses and also help in understanding the human relevance of preclinical toxicities. Such experiments are only possible if TCs are developed using animal and non-animal (i.e. human) cells.14
We also acknowledge a growing need to study not only small molecules but also the emerging new drug modalities (e.g. monoclonal antibodies, protein therapeutics, antibody-drug conjugates, and other biotherapeutic modalities). Current approaches to investigate on and off target pharmacology, safety liability, and disposition of these modalities is often limited to in vivo preclinical studies in large animals that share binding affinity (e.g. non-human primates) with the human patients (i.e. the molecules are humanized). Few in vitro systems provide the opportunity to study the cell, tissue and organ-level fate of these more complex molecules. Accordingly, molecular design is largely predicated on target binding affinity alone rather than cell-specific trafficking or pharmacodynamics, and safety assessment is delayed until very labor, cost and animal-intensive studies late in preclinical development. This is due to the complexity associated with large molecule disposition and therapeutic activities, especially monoclonal antibodies, in which they often require extravasation to reach the target cell/tissues and the degree of extravasation and tissue accumulation is dependent upon the tissue/organ system and post-translational modifications (e.g. glycosylation) on the protein.15 Therefore, human-relevant organ-based TCs, in particular those that include both vascular and tissue components, would offer significant opportunity to design complex biological therapeutics optimized for tissue penetration, target engagement, accumulation, and catabolism in a relevant biological context.
The ability to perform a morphological assessment is also attractive when assessing structural integrity and drug- or disease-induced structural changes. Application of non-invasive sensors and imaging modalities would be advantageous features while real-time sampling of liquid and cellular components would be key to characterizing and assessing circulating biomarkers.
Performance characterization
Confidence in the outputs of microphysiological systems will be substantially increased by representation of relevant in vivo biology, responses to injury, pharmacology, and an ability to relate in vitro cellular and organ level biology to more integrated and phenotypic in vivo responses. The level of fidelity to which these various features are demonstrated or represented is very much dependent on the context of use for the platform as described above.
Characterization of basic TC microanatomy, physiology, pharmacology and response to injury is imperative to establishing the biological relevance of these platforms. For example, the microanatomy of a TC representing the gastrointestinal tract for safety screening or drug absorption should look to mirror the structural characteristics of the appropriate epithelium through the inclusion of enterocytes, paneth cells, stem cells, and goblet cells at relevant in vivo proportions. Exhibiting critical molecular and biochemical features including expression and/or activity of transporters and drug metabolizing enzymes would also be important. A more mechanistic platform to evaluate a specific cellular interaction might need to be less complex and thus allow a simpler, higher throughput system. It may be impossible to replicate all of the necessary features in a single chip, thus requiring the ability to integrate or collate data gathered from complementary systems. Application of clinically relevant or translational biomarkers may also be useful to provide a line of sight from in vitro to in vivo biological responses.
Defining superiority to current capabilities will also be important. Taking the liver as an example, two-dimensional cell models are used successfully to screen for direct hepatocellular toxicities as well as drug metabolism. They are amenable to high throughput screening, return good positive predictive values of direct injury and are capable of detecting metabolite driven toxicities.16 But there is a rapid loss of “liver” phenotype during culture and they are not amenable to repeat-dose studies. As only parenchymal cells are present, a degree of fidelity and cellular interaction is lost which may ultimately impact on our desire to improve clinical translation.17
For drug development applications, the ability of TCs to model drug–cell interactions and induce pharmacology or toxicity is critical. A myriad of well-characterized pharmacologic and toxic compounds are available and can be used to demonstrate in vivo-like responses in these novel platforms. For example, an airway smooth muscle chip should demonstrate the ability to contract in the presence of histamine and relax in response to beta agonists such as formoterol. An important role for pharmaceutical scientists is to identify organ-specific tool compounds, in vivo exposure concentrations and expected responses. They may also need to provide proprietary compounds to demonstrate pharmacology and toxicology of contemporary interest. TCs should be able to demonstrate clear pharmacological responses delineated by concentration–response curves over a relevant concentration range. For example in a renal TC, there should be a concentration-related increase in diuresis in response to increasing concentrations of a diuretic, and in a cardiac TC, there should be a decrease in beat rate in response to a calcium channel blocker.
Another key enabler for building confidence in in vitro systems will be our ability to relate more mechanistic and molecular endpoints to their likely clinical manifestations or outcomes. Though changing rapidly, modern clinical medicine is still largely rooted in the characterization of phenotypes (e.g. the cardinal clinical signs of inflammation or measurable changes in organ function). We will also need to be able to distinguish species-specific responses (i.e. does response in a human-relevant in vitro system supersede a response in a whole animal system or vice versa?). Taking the cardiovascular system as an example, cardiac contractile force in vivo can be assessed by measuring left ventricular pressure with a surgically implanted pressure catheter (as it is in some animal studies) or ejection fraction and fractional shortening with echocardiography. Changes in cardiac contractility may result from direct effects on the cardiomyocyte (e.g. digitalis increases intracellular calcium), effects on downstream vascular tone (vasodilators decrease downstream resistance/afterload), and changes in blood volume (preload) or signaling from the autonomic nervous system. Additionally, changes in any one of those influences are often compensated by changes in the others. In contrast, in vitro systems are generally composed only of cardiomyocytes and measure cellular contractile force, cellular shortening, intracellular calcium flux or changes in impedance (surrogate for changes in cellular conformation). Vascular, fluid volume and autonomic influences would not be represented. Also, changes in cellular physiology (e.g. how much of a change in calcium flux would equate to some measurable change in ejection fraction?) would need to be related to expected changes in whole organ or circulatory system function with consideration for the compensatory changes. In the context of integrative in vivo physiology, in vitro to in vivo extrapolation is challenging.
Analytical validation
Aside from biological characterization, there is a need to establish analytical performance standards which should be the responsibility of the end-user (i.e. pharmaceutical industry). Key aspects to validate include throughput capability, biological platform stability, drug-biomaterial interactions, intra- and inter-laboratory reproducibility, integration and compatibility with existing laboratory processes and feasibility of shipping these delicate systems between vendors and users. Third party Testing Centers (https://ncats.nih.gov/tissuechip/projects/centers) recently proposed and funded by NCATS are an opportunity to conduct robust analytical validation required by the pharmaceutical industry without distracting the academic innovators who continue to refine their platforms. These testing centers will test and validate TC platforms independently; determine portability, reproducibility and robustness of TC technology as developed by the TC developers, particularly for use by regulatory agencies and pharmaceutical companies; and promote adoption of this technology by the broad research community through creation of a publicly accessible database of TC-based pre-clinical outcomes from a well-defined set of reference validation compounds. The reference set is specific for each organ or tissue platform and is compiled based on known performance metrics and technical specifications provided by the TC developers, and input from IQ and FDA on the most appropriate compounds, biomarkers and assays. A formal qualification of TC models to enable drug regulatory approvals may be an eventual interest but in the spirit of partnership early engagement with regulatory authorities will be key.
Commercialization
The ultimate uptake and impact of TCs depends on the ability to industrialize or scale up their manufacture and distribution as well as attain some level of commercial sustainability. This sustainability could be the product of the right partnerships throughout the TC development and testing process. As illustrated, these platforms will clearly have wide application in drug discovery which will require their consistent supply either as a product that organizations can purchase and use in their laboratories or a service that is outsourced. Broad application as a screening strategy which would facilitate more rapid uptake and confidence-building is more likely to come as a product than a service. Mechanistic applications might be sustainable as a fee-for-service. These two contexts of use have very different long-term development implications impacting the biological and analytical complexity of the systems, the throughput, cost and even the engineering infrastructure needed to support them. In a rapidly developing area of technology, these are critical enablers that are often neglected.
Conclusion
TCs are uniquely poised to fill the need for improved human models and will enable investigators to develop a deeper understanding of drug responses in different genders, genetic backgrounds and disease phenotypes. Minimizing the leaps of faith between “healthy” human non-physiological in vitro models, healthy and “disease” animal models and the eventual human patient will no doubt offer a distinct advantage to the discovery of new therapeutics by avoiding late detection of safety liabilities. The speed of innovation in this field is rapid and the possibilities seem endless. But, in order to help deliver the benefits of this technology, a robust characterization, validation, and industrialization process must be defined, agreed, and implemented. This must involve partnership between the innovators and end users as well as significant stakeholders such as the regulatory authorities. This article has set out the challenges, scripted a progression strategy, and produced a call to action for each party involved so now it really is time to sharpen our pencils and turn scientific fiction into scientific fact.
Acknowledgements
The authors would like to thank Kyle Brimacombe for his assistance in creating the chip image that encapsulates the key themes of this manuscript and Rhiannon David for her assistance in creating the context of use image.
Authors’ contribution
All authors participated in the discussions and debates that built the content of this manuscript and also each reviewed the completed article; LE, KF, and BB wrote the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol 2014; 87: 162–71. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014; 32: 760–72. [DOI] [PubMed] [Google Scholar]
- 3.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 2015; 14: 248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thornloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Trans Med 2014; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014; 20: 616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Lia H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci USA 2015; 113: E7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laverty HG, Benson C, Cartwright EJ, Cross MJ, Garland C, Hammond T, Holloway C, McMahon N, Milligan J, Park BK, Pirmohamed M, Pollard C, Radford J, Roome N, Sager P, Singh S, Suter T, Suter W, Trafford A, Volders PGA, Wallis R, Weaver R, York M, Valentin J-P. How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines? Br J Pharmacol 2011; 163: 675–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge BR, Herman G, Van Vleet J. Cardiac, Vascular, and skeletal muscle systems. In: Haschek W, Rousseaux C, Wallig M. (eds). Haschek and Rousseaux’s handbook of toxicologic pathology, 3rd ed NY: Elsevier Academic Press, 2013, pp. 1567–1657. [Google Scholar]
- 9.Materne E-M, Maschmeyer I, Lorenz AK, Horland R, Schimek KMS, Busek M, Sonntag F, Lauster R, Marx U. The multi-organ chip – a microfluidic platform for long-term multi-tissue coculture. J Vis Expt 2015; 98: e52526 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oleaga O, Bernabini C, Smith AST, Srinivasan B, Jackson M, McLamb W, Platt V, Bridges R, Cai Y, Santhanam N, Berry B, Najjar S, Akanda N, Guo X, Martin C, Ekman G, Esch MB, Langer J, Ouedraogo G, Cotovio J, Breton L, Shuler ML, Hickman JJ. Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep 2016; 6: 20030–20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton DM. Importance of species selection in drug toxicity testing. Toxicol Lett 1998; 102: 545–50.. [DOI] [PubMed] [Google Scholar]
- 12.Li AP. Accurate prediction of human drug toxicity: a major challenge in drug development. Chem Biol Interact 2004; 150: 3–7. [DOI] [PubMed] [Google Scholar]
- 13.Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2006; 2: 875–94.. [DOI] [PubMed] [Google Scholar]
- 14.Haney S, Jones B, Andersson L, Kodela K, Ewart L, Hamilton G. Development of rat, dog, and human liver-on-chip models that maintain long-term viability and CYP450 enzyme activity levels. 2015; Abstract at ISSX Florida meeting. Available at: http://issx.confex.com/issx/20NA/webprogram/Paper35184.html.
- 15.Venetz D, Hess C, Lin CW, Aebi M, Neri D. Glycosylation profiles determine extravasation and disease-targeting properties of armed antibodies. Proc Natl Acad Sci 2015; 112: 2000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schadt S, Simon S, Kustermann S, Boess F, McGinnis C, Brink A, Lieven R, Fowler S, Youdim K, Ullah M, Marschmann M, Zihlmann C, Siegrist YM, Cascais AC, Di Lenarda E, Durr E, Schaub N, Ang X, Starke V, Singer T, Alvarez-Sanchez R, Roth AB, Schuler F, Funk C. Minimizing DILI risk in drug discovery – a screening tool for drug candidates. Toxicol In Vitro 2015; 30(1 Pt B): 429–37. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland JJ, Jolly RA, Goldstein KM, Stevens JL. Assessing concordance of drug induced transcriptional response in rodent liver and cultured hepatocytes. PLoS Comput Biol 2016; 12: e1004847–e1004847. [DOI] [PMC free article] [PubMed] [Google Scholar]