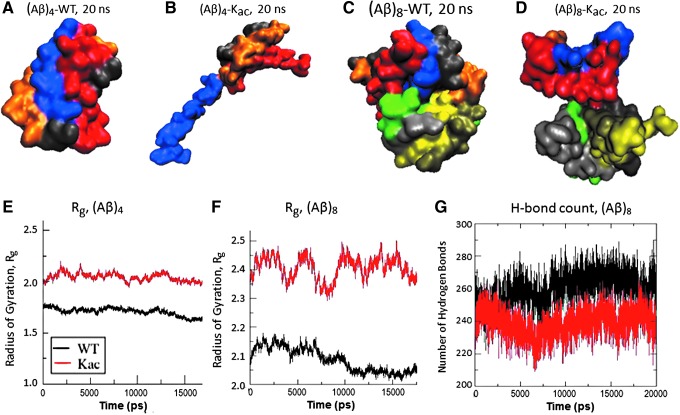
FIG. 11.
Acetylation reduces compactness and stability of Aβ1–42 oligomers. Molecular docking models and molecular-dynamic simulations indicate that lysine acetylation in Aβ1–42 (“Kac”) destabilizes its assembly into tetramers and octamers. Optimized docking models are shown for: (A) native Aβ1–42 tetramer, (B) acetylated Aβ1–42 tetramer, (C) native Aβ1–42 octamer, and (D) acetylated Aβ1–42 octamer. Molecular-dynamic simulations in GROMACS indicate increased dispersion (radius of gyration) for acetylated tetramer (E) and octamer (F), and decreased hydrogen bonding for acetylated octamer (G), relative to their unmodified forms. (E–G) Properties of lysine-acetylated oligomers (Kac) are shown in red, and those of unmodified Aβ oligomers are indicated in black.