Figure 1. Flow of Patients Through the Study.
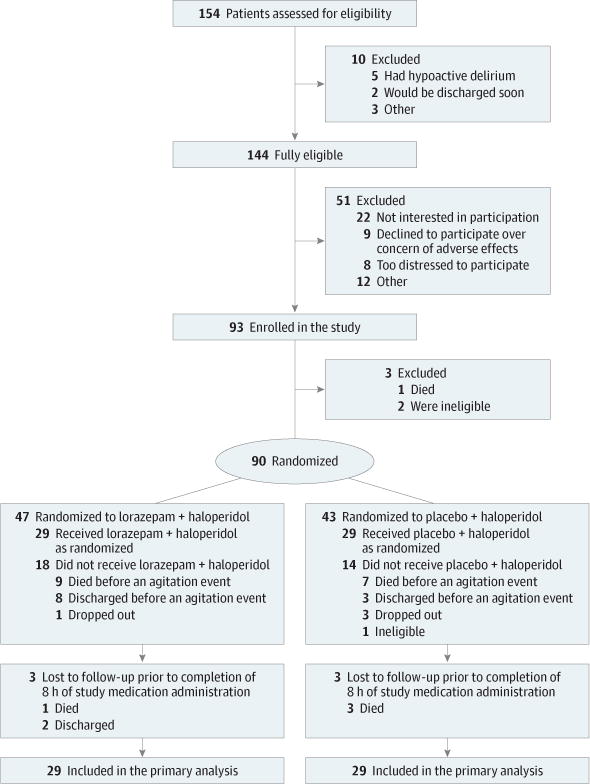
At the time of enrollment, patients were randomized to lorazepam or placebo. All enrolled patients immediately began a standardized regimen with haloperidol 2 mg every 4 hours intravenously and 2 mg every hour as needed for agitation. Because of the fluctuating nature of delirium, the Richmond Agitation-Sedation Scale score of each patient was monitored every 2 hours until the patient’s score was 1 or more and required rescue medication per the judgment of the bedside nurse. Once the dose of haloperidol was increased and standardized, 27 of 90 randomized patients (30%) did not develop further agitation until death or discharge and thus did not require the study medication.
