Figure 3. TMEM258 Interacts with the OST Complex and Regulates Protein N-linked Glycosylation.
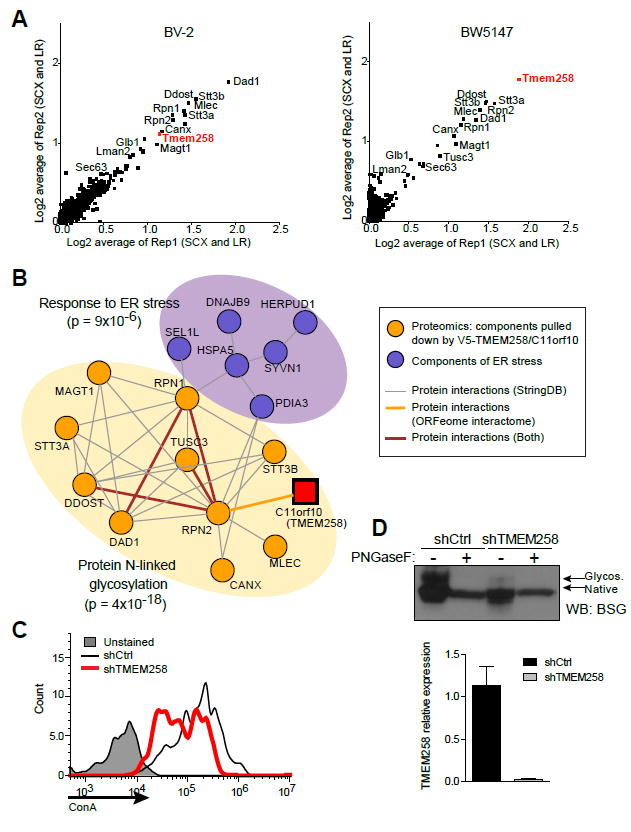
(A) TMEM258 interaction partners were identified in BV-2 cells (left) and BW5147 cells (right) expressing V5-tagged TMEM258. High-performance liquid chromatography-mass spectrometry was employed to detect proteins after immunoprecipitation with anti-V5 antibody.
(B) Network analysis integrating TMEM258 interaction partners with the OST complex and ER stress response.
(C) TMEM258 was knocked down in HeLa cells, and glycoproteins were detected by surface staining with FITC-labeled concanavalin A (ConA) followed by FACS.
(D) Measurement of N-linked glycosylation on the prototypical glycoprotein basigen (BSG) was monitored by western blot. Where indicated, samples were deglycosylated with PNGaseF. Knockdown of TMEM258 in HeLa cells was verified by qPCR. shCtrl, control shRNA. Data represent mean and SD.
See also Figure S2.
