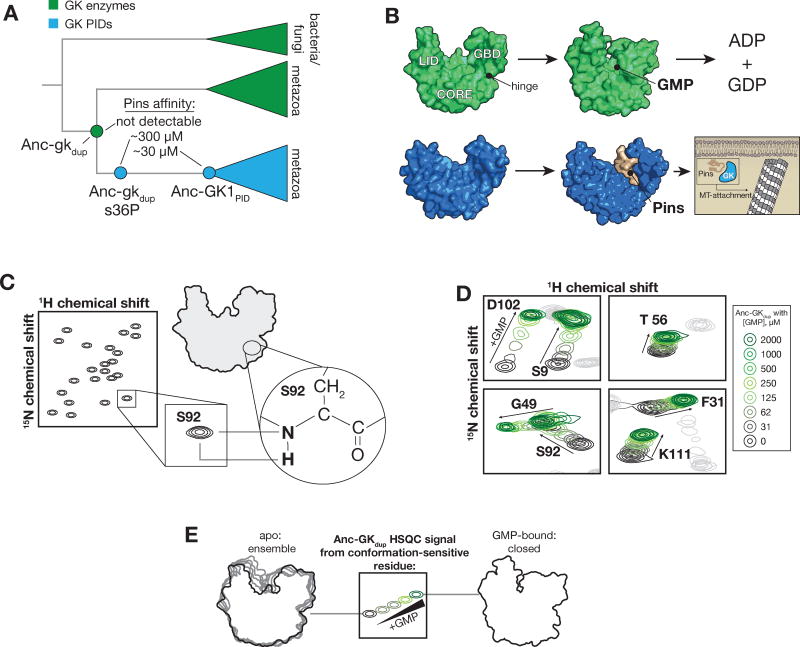
Figure 1.
Characterization of ancestral guanylate kinase conformational equilibria. A. Reduced phylogeny of the protein family containing gk enzymes (green) and protein-binding GKPIDs (blue). Mutation of the ancestral serine 36 to proline (Anc-gkdup s36P) abolishes catalytic activity and confers moderate protein binding affinity. The affinities for Pins are shown (ND = not detected)6. B. Extant GK enzymes (green) are primarily in an open conformation until nucleotides bind, at which point they close to position ATP and GMP phosphates for catalysis. The conformational change involves rotation of the GMP binding domain (GBD) towards the “CORE” and ATP-binding “LID”. A “hinge” composed of residues that connect the core and GBD mediates rotation. The extant GK domain (blue) from Discs large (Dlg) binds phosphorylated Partner of Inscuteable (Pins) during cortically-directed mitotic spindle orientation in metazoa. The PDB IDs for the structures used throughout are as follows: GK enzyme open conformation (1EX6, chain A), GK enzyme closed conformation (1EX7), s36P GK enzyme open conformation (4F4J), apo GK PID (1KJW), Pins-bound GK PID (3TVT). C. 1H-15N HSQC NMR experiment. Signals are observed for nitrogen atoms bonded to hydrogens, primarily peptide bonds (except for proline which does not have a hydrogen atom at this position). The position of the signal is dependent on the chemical shift of both atoms, which is in turn sensitive to structure. D. GMP binding induces linear changes in the chemical shifts of GK residues consistent with GK closing. The 1H-15N HSQC NMR signals for several GK residues that don’t directly contact GMP are shown when GMP concentrations are gradually increased (concentration key shown at right). The full spectra are shown in Supporting Information. E. Origin of GK 1H-15N HSQC NMR signals and the effect of GMP. An example signal that is influenced by GMP binding is shown. The GK exists as a structural ensemble in the absence of nucleotide (see Figure 3) so the HSQC signals from many GK residues are the average of many conformations. GMP-binding shifts the conformation to a more homogenous closed conformation. HSQC signals can be influenced directly by GMP (for residues near the binding site) and/or through the indirect effects on GK conformation. The signal at intermediate, non-saturating concentrations of GMP results from averaging of the relatively few closed and many open conformations.