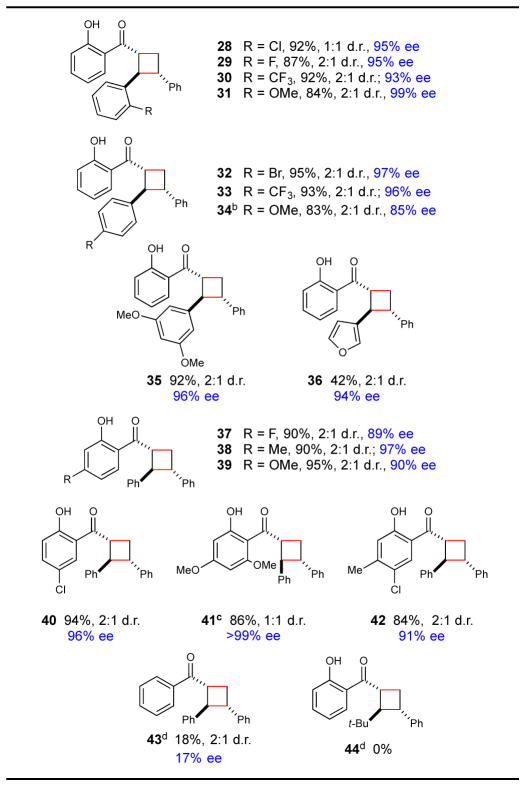
Table 3.
Reaction scope with respect to chalcones.a
Isolated yields are the averaged results of two reproducible experiments. Diastereomer ratios were determined by 1H NMR analysis of the unpurified reaction mixtures. Enantiomeric excesses for the major diastereomer were determined by chiral SFC. See Supporting Information for details.
Reaction was irradiated with monochromatic high-intensity blue LED.
Reaction was irradiated for 40 h.
Yields determined by 1H NMR analysis using phenanthrene as a calibrated internal standard.