Figure 5. Characterization of the anti-CD133/anti-EGFRvIII bi-specific antibody (BsAb).
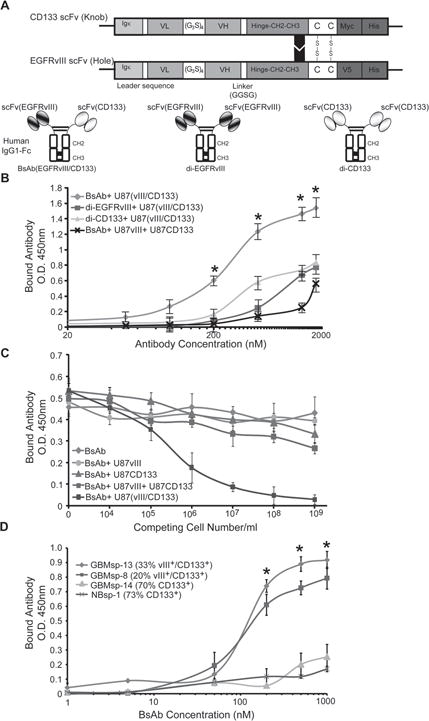
A) Schematic of the BsAb showing scFv chains of anti-CD133 and anti-EGFRvIII. Igκ = leader sequence from kappa chain of human IgG1, VL = variable light chain, (G3S)4 = hinge peptide, VH = variable heavy chain, CH2-CH3 = Fc domain from human IgG1, Myc, V5 and His = tags for purification and identification. Di-valent anti-CD133 (di-CD133) and anti-EGFRvIII (di-EGFRvIII) antibodies were also created. B) ELISA dose-response analysis of the BsAb ( ), di-EGFRvIII ( ), and di-CD133 ( ), reagents on U87 cells co-transfected with EGFRvIII and CD133, and analysis of the BsAb reagent on an equal mix of cells transfected with either EGFRvIII or CD133 (x). * = statistical significance (p < 0.001). C) Competitive sandwich ELISA to identify specificity of BsAb towards EGFRvIII+/CD133+ cells. BsAb was not pre-incubated ( ) or pre-incubated with U87 cells expressing either ( ) CD133, ( ) EGFRvIII, or both proteins expressed in the same cell (EGFRvIII/CD133) ( ), or a pool of cells expressing CD133 or EGFRvIII ( ) prior to incubation with membrane fractions from U87-EGFRvIII/CD133 cells (105cells/well). D) Live cell immunoprecipitation and ELISA analysis of BsAb binding to GBM tumor spheres in cultures with variable EGFRvIII and CD133 expression. ( ) GBMsp-13, ( ) GBMsp-8, ( ) GBMsp-14, (x) NBsp-1. * = statistical significance (p<0.001). Data points are represented as mean ± SEM of three individual experiments. See also Figure S3.
