Figure 6. BsAb eliminates EGFRvIII+/CD133+cells from culture, enhances cytotoxicity, impairs GBMsp tumorigenicity, and enhances survival.
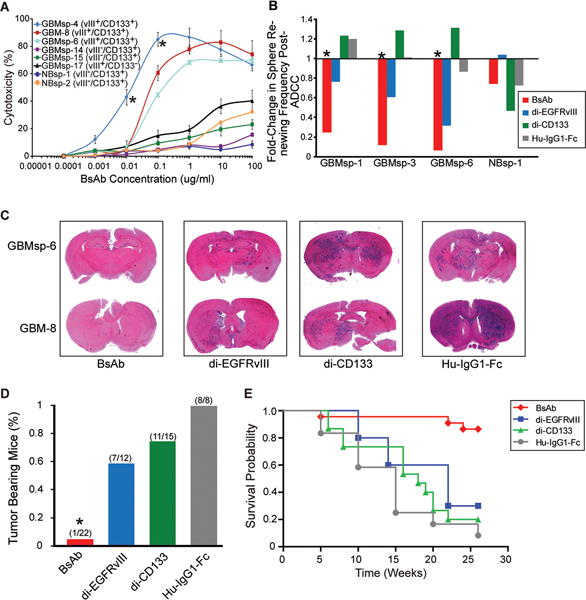
A) BsAb Antibody-Dependent Cell Mediated Cytotoxicity (ADCC) on primary GBM cells (GBM-8), GBM tumor spheres (GBMsp), and normal neurospheres from epileptic patients (NBsp). All experiments were carried out in triplicate. Error bars represent ± SEM. *= statistical significance (p<0.001). B) Fold-change in the frequency of NBsp and GBMsp initiating cells pre and post-ADCC. Data represented as change in sphere renewing frequency, with and without the presence of BsAb (red) di-EGFRvIII (blue), di-CD133 (green) and human-IgG1-Fc (grey). C) Antibodies were mixed with unsorted cells (100,000 total) from EGFRvIII+/CD133+ tumors, injected into the brains of NOD-SCID mice, and assayed after 26 weeks. Coronal sections from mouse brain were stained with H&E. D) Percent tumor bearing mice from four human glioma samples (GBMsp-4, GBMsp-6, GBM8 and GBM12) co-injected with the various antibodies. Mice were analyzed after 26 weeks. E) Kaplan Meier survival probability shows the BsAb significantly enhances survival (p<0.0001) as compared to the mono-specific antibodies. See also Figures S4 and S5.
