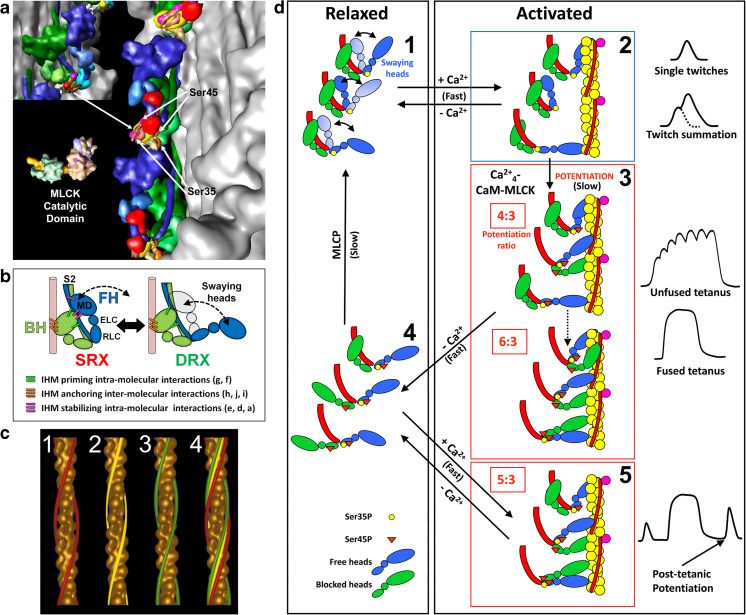
Fig. 7.
Functional implications of the tarantula asymmetric IHM 3DTP model on the relaxation and activation of thick filaments. a The IHM asymmetric structure 3DTP (inset) produces different environments for the myosin regulatory light chain (RLC) NTEs of the free (blue) and blocked (green) heads containing phosphorylatable Ser35 and Ser45. This enables the MLCK to access only the serines of the free head. b The constitutively mono-phosphorylated Ser35 on the free heads are ready to interact with the Ca2+-switched on thin filament through the free head swaying mechanism involving the formation and disruption of intramolecular stabilizing interactions (magenta bars) between the swaying free head and the blocked head and interconnecting intermolecular interactions (Fig. 9b). The un-phosphorylated blocked head is docked onto its own S2 by priming intramolecular interactions (green bars) and with its neighbor myosin tail with anchoring intermolecular interactions (orange bars) (Alamo et al. 2016). c Tarantula thin filament is Ca2+- activated according to the steric model of muscle contraction (Huxley 1973) shown by tropomyosin (TM) movements (c4) on thin filament 3D–reconstructions in relaxed (c1, TM strands, red), activating (c2, yellow) and rigor (c3, green). d Model for activation (d1–d2), potentiation (d2–d3), post-tetanic potentiation (d3–d5) and relaxation (d2–d1 and d4–d1) for tarantula striated muscle dual thin and thick filaments regulation. (a) and (d) reproduced with permission from Brito et al. (2011) and the Journal of Molecular Biology, (c) reproduced with permission from Craig and Lehman (2001) and the Journal of Molecular Biology