Fig. 2.
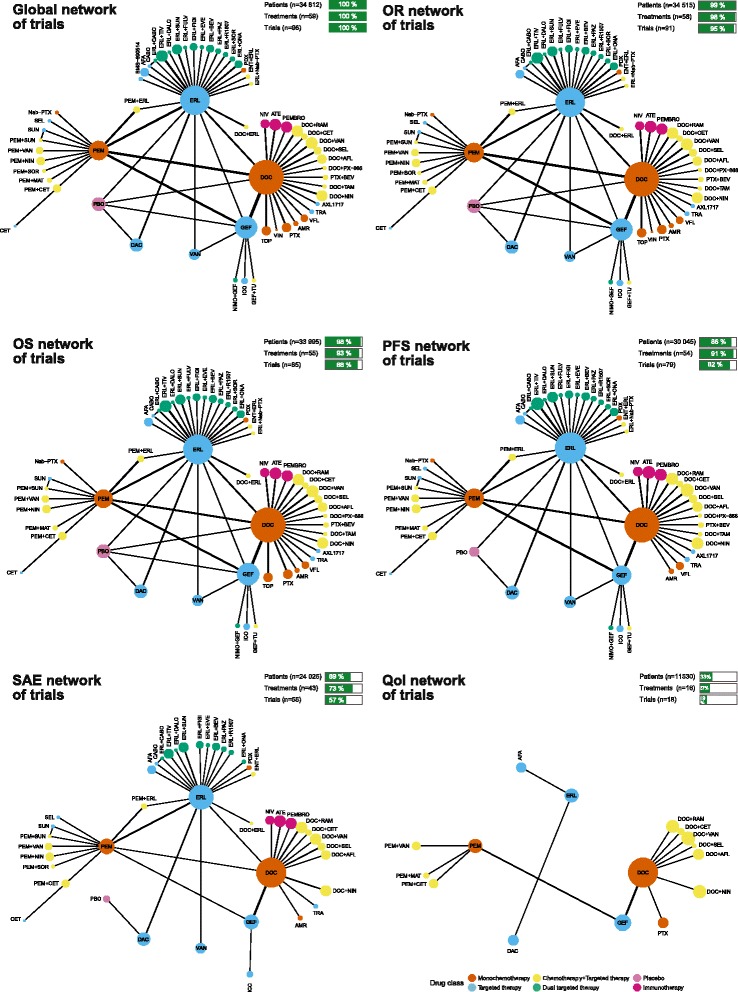
Network graphs of trials assessing second-line treatments in advanced NSCLC with wild-type or unknown status for EGFR for all eligible trials, ObR, OS, PFS, SAEs, and QoL. The five trials with chemotherapy (i.e., docetaxel or pemetrexed) at the investigators’ discretion and the HANSHIN trial were excluded from these networks. The thickness of the lines is proportional to the number of trials evaluating each comparison. The size of the nodes is proportional to the number of patients allocated to the corresponding treatment. AFA afatinib, AFL aflibercept, AMR amrubicin, ATE atezolizumab, BEV bevacizumab, CABO cabozantinib, CET cetuximab, DAC dacomitinib, DALO dalotuzumab, DOC docetaxel, ENT entinostat, ERL erlotinib, EVE everolimus, FIGI figitumumab, FULV fulvestrant, GEF gefitinib, ICO icotinib, MAT matuzumab, Nab-PTX nab-paclitaxel, NIMO nimotuzumab, NIN nintedanib, NIV nivolumab, ONA onartuzumab, PAZ pazopanib, PBO placebo, PDX pralatrexate, PEM pemetrexed, PEMBRO pembrolizumab, PTX paclitaxel, RAM ramucirumab, SEL selumetinib, SOR sorafenib, SUN sunitinib, TAM tamoxifen, TIV tivantinib, TOP topotecan, TRA trametinib, TU tegafur-uracil, VAN vandetanib, VFL vinflunine, VIN vinorelbine
