Fig. 1b. Number of new molecular entities (NMEs) and Biologics License Applications (BLAs) approved by the Center for Drug Evaluation and Research (CDER) from 2006 to 2015.
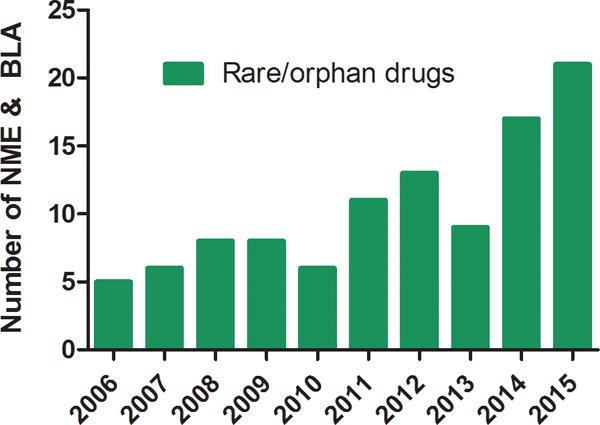
Data are from the FDA website (http://www.accessdata.fda.gov/scripts/cder/daf/).
