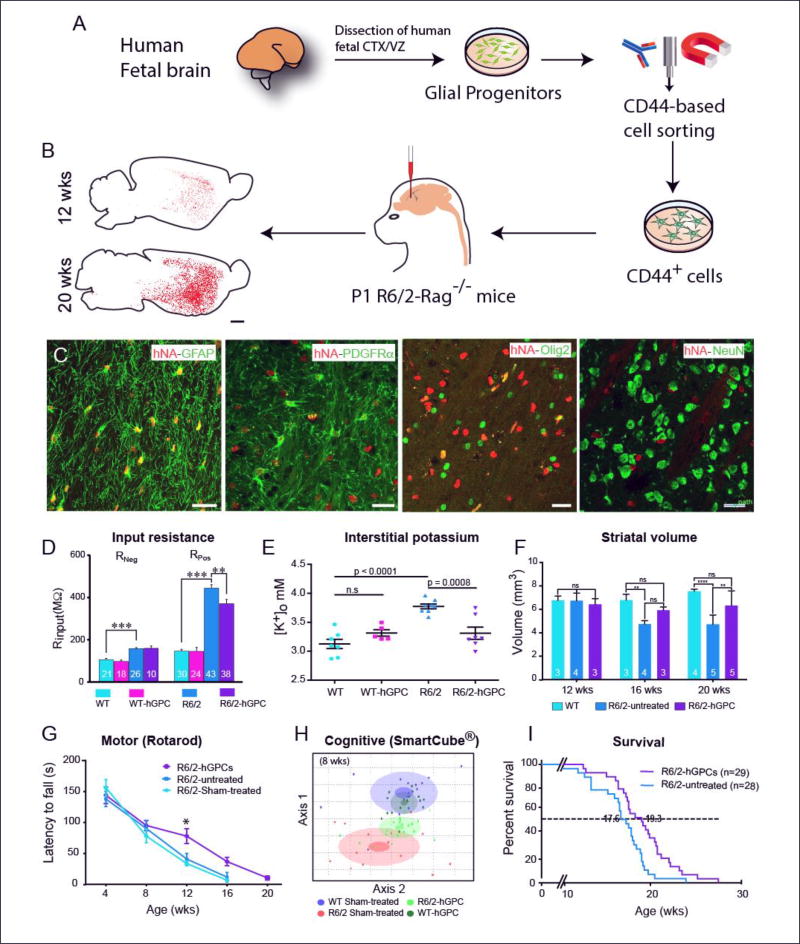
Figure 3. Glial progenitor grafts slow disease progression, stabilize neuronal excitability and extend survival in mouse models of Huntington disease.
A, Schematic outlines the preparation and delivery of normal CD44-defined astroglial progenitor cells into the striata of neonatal HTT mutant R6/2 (120Q) × rag1−/− mice. B, CD44-sorted hGPCs colonized the R6/2 × rag1−/− striatum Striatal engraftment of the R6/2 mice by CD44-sorted hGPCs was robust and dense. Donor-derived cells increased as a function of time, such that transplanted hGPCs expanded to largely replace host glia in the striata and ventral forebrains of recipient R6/2 mice by 20 weeks. Scale: 1 mm. C, By 20 weeks after neonatal graft, the donor hGPCs (human nuclear antigen, red) integrated as astrocytes (GFAP, green) or persisted as GPCs (PDGFαR and olig2, green), but did not give rise to neurons; no overlap was seen of hN and neuronal NeuN. Scale: 25 µm.
D. Chimerization with normal glia partially normalized MSN physiological function. Whole-cell I-clamp recordings from rag1−/− wild-type, CD44 hGPC-engrafted rag1−/− wild-types, R6/2 × rag1−/− mice, and CD44-engrafted rag1−/− mice revealed that the input resistance Rinput, was significantly higher in R6/2 × rag1−/− striatal neurons than in wild-type × rag1−/− controls, but was partially restored to normal in R6/2 mice chimerized with normal CD44-sorted hGPCs. E. Normal glial engraftment reduces interstitial K+ levels in the R6/2 striatum Potassium electrodes were used to measure the interstitial levels of striatal K+ in both wild-type mice and R6/2 littermates at 16 weeks of age (± 4 days), with and without neonatal intrastriatal transplants of CD44-sorted hGPCs. Untreated R6/2 mice manifested significantly higher levels of interstitial K, which were restored to normal in R6/2 mice neonatally engrafted with hGPCs. In contrast, hGPC engraftment did not influence the interstitial K+ levels of wild-type mice. F. Striatal involution of R6/2 mice was slowed by normal glial engraftment The neostriata of R6/2 HD mice typically shrink in volume with age. Human GPC engraftment attenuated this process, in that R6/2 mice manifested larger striatal volumes than unengrafted R6/2 mice by 16 weeks of age, with preservation of R6/2 striatal volumes at levels no different than wild-type controls. E–F, Means ± SEM; ** and ***, p< 0.01, and 0.0001, 1-way ANOVA.
G. Chimerization with normal glia slows motor loss and extends survival of R6/2 mice Linear regression revealed that the rate of rotarod-assessed motor deterioration of R6/2 mice was significantly slower in mice engrafted with human GPCs than in untreated mice (p<0.001 by ANOVA). H. Treatment with hGPCs slowed cognitive decline in R6/2 mice SmartCube testing (Psychogenics), a multimodal assessment of cognitive function (Benraiss et al., 2016), revealed a significant difference between sham-treated wild-type (WT) and R6/2 mice by 8 weeks of age, with significant functional preservation in the R6/2 mice when treated neonatally with normal hGPCs. In H, each dot represents a mouse. The center, small and large ellipses represent the mean, standard error and standard deviation of the composite features for each group. See (Benraiss et al., 2016) for detail. I. R6/2 mice whose striata were engrafted with human GPCs survived significantly longer than unengrafted mice (p<0.01, Mantel-Cox Log-rank test).