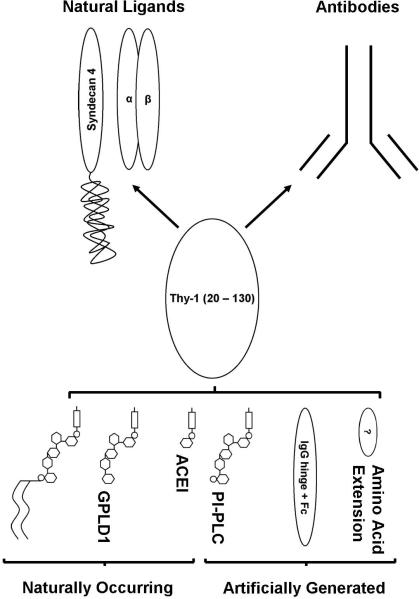
FIGURE 7. Diagram of GPI anchored, delipidated, and recombinant Thy-1 depicted with relative affinity to antibodies raised against GPI-anchored and known ligands of Thy-1.
Delipidation induces a change in the conformation of Thy-1 causing it to lose affinity for antibodies that recognize the native conformation of THY1 with a complete GPI-anchor. Recombinant soluble Thy-1, i.e. expressed without a GPI-attachment signal, also has a conformation that is not recognized by these same antibodies. However, some forms of recombinant soluble Thy-1 with either a specific AA extension or possibly omission of certain AAs, i.e. CYS 130, may restore affinity. This phenomenon is depicted in the upper half of the diagram, in which white ovals represent the mature Thy-1 polypeptide and narrow black ovals are AA extensions off CYS 130. The conformation assumed by Thy-1 released from cell surfaces with GPLD1 and ACE with respect to antibody affinity, is not known. Moreover, the effect conformational differences may have on the affinity for known natural ligands or whether new ones are gained is not known. All are important to consider when raising antibodies to, using a recombinant form of, or examining the function of Thy-1.