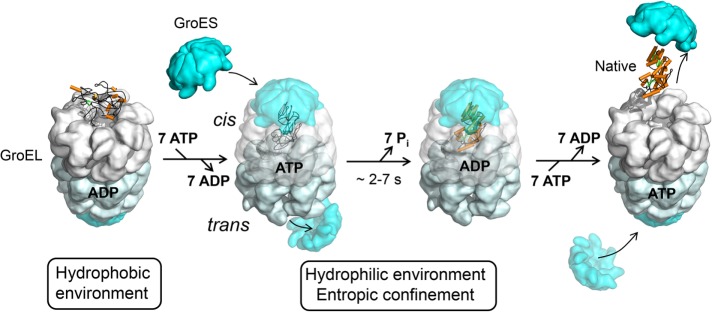
FIGURE 2:
The GroEL/GroES reaction cycle. The current model for protein folding in the chaperonin cage is shown. Substrate binding to GroEL may result in local unfolding (Sharma et al., 2008). ATP binding then triggers a conformational rearrangement of the GroEL apical domains. This is followed by the binding of GroES (forming the cis complex) and substrate encapsulation for folding. At the same time, ADP and GroES dissociate from the opposite (trans) GroEL ring, allowing the release of substrate that had been enclosed in the former cis complex (omitted for simplicity). Substrate remains encapsulated, free to fold, for the time needed to hydrolyze the seven ATP molecules in the newly formed cis complex (∼2–7 s, dependent on temperature). Folding inside the cage may be accelerated due to entropic confinement of dynamic folding intermediates. Binding of ATP and GroES to the trans ring causes the opening of the cis complex. Diagram reproduced from Hartl et al. (2011).