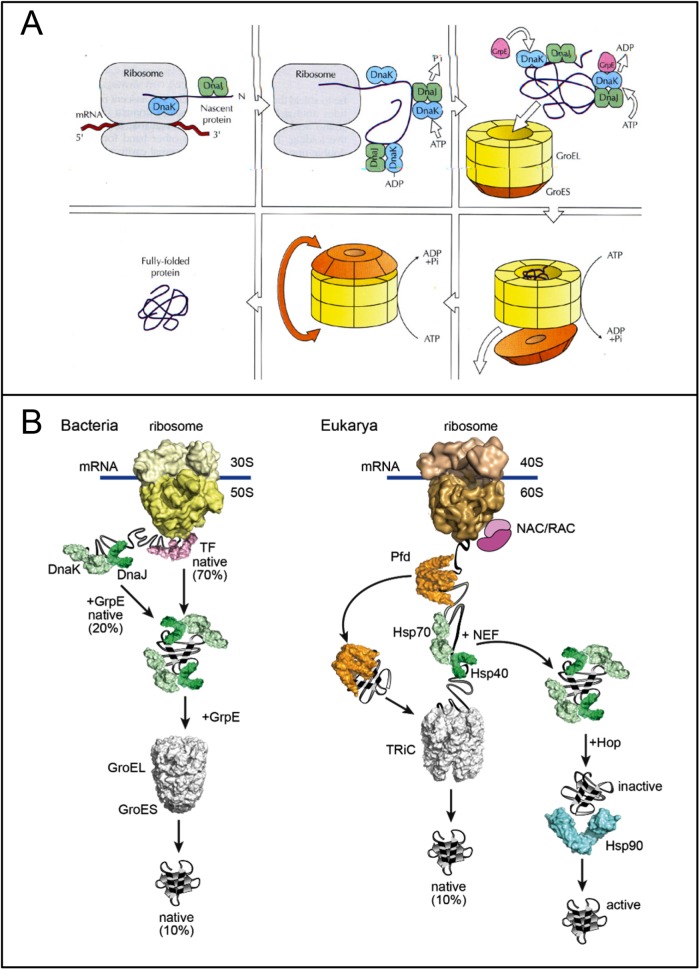
FIGURE 3:
Chaperone pathways in the bacterial and eulkaryotic cytosol. (A) Early model for the E. coli cytosol, shown for a GroEL-dependent protein (reproduced from Martin and Hartl, 1993). In clockwise direction: The nascent chain is stabilized in a folding-competent state during translation by the Hsp70 chaperone system (DnaK/DnaJ). These chaperones bind hydrophobic segments exposed by the extended chain that will later be buried within the folded structure. Upon completion of translation, the protein is unable to fold with DnaK and DnaJ and must be transferred into the central cavity of GroEL. This step requires GrpE, the nucleotide exchange factor of DnaK. Following binding of the protein in a “molten globule”–like conformation into the open ring of GroEL, the protein is encapsulated by GroES in the folding cage. The model was later extended to include the cooperation of DnaK with the ribosome-bound chaperone Trigger factor (Deuerling et al., 1999; Teter et al., 1999) and the finding that the Hsp70 system mediates the folding of proteins that do not require the physical enmvironment of the chaperonin cage (Szabo et al., 1994). (B) Current models for the folding pathways in the bacterial and eukaryotic cytosol reproduced from Balchin et al. (2016). NAC/RAC are eukaryotic ribosome-binding chaperones with a function similar to that of bacterial Trigger factor (TF). Prefoldin (Pfd) recruits the chaperonin TRiC to certain nascent chains. Like TRiC, the Hsp90 chaperone system functions downstream of Hsp70. Hop mediates contacts between Hsp70 and Hsp90.