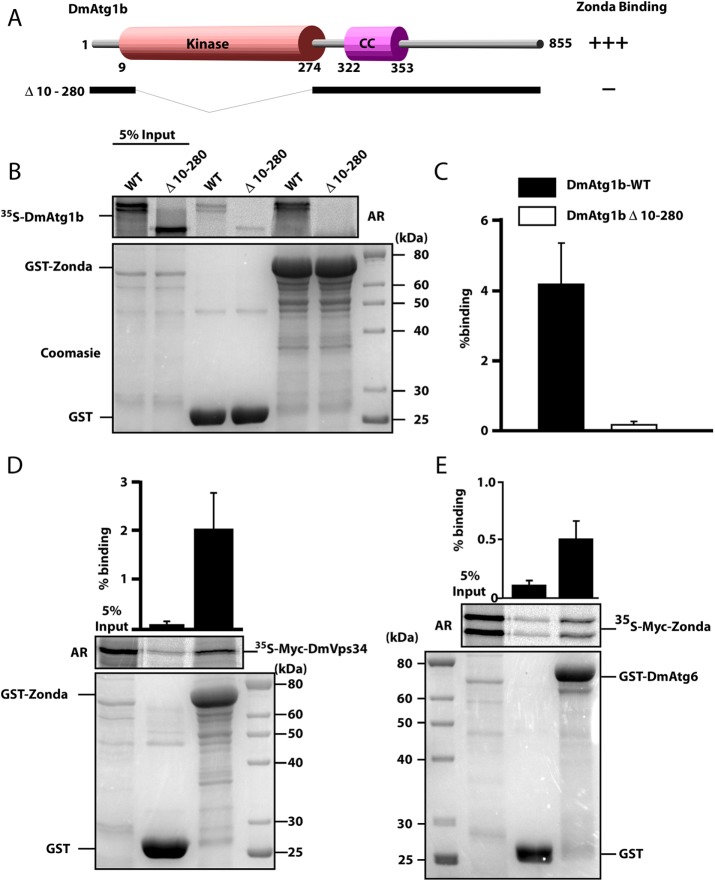
FIGURE 4:
Zda binds directly to Atg1B, Atg6, and Vps34 in vitro. (A) Schematic representation of the DmAtg1b domain structure and deletion constructs made to determine domain-mediated binding to Zda. (B) GST pull-down assay using in vitro–translated 35S-labeled Myc-tagged Dm-Atg1B. In vitro–translated full length, or a kinase domain deletion mutant, of Myc-tagged DmAtg1B were incubated with recombinant GST or GST-Zda expressed in E. coli attached to glutathione–sepharose beads and bound protein was detected by autoradiography (AR). The Coomassie-stained SDS–PAGE gel indicates the amount of GST protein used in the assay. The graph displays percentage binding of in vitro–translated protein relative to the input. (C) Similar to B except that in vitro–translated 35S-labeled Myc-tagged Zda was incubated with GST or GST-DmAtg6. Quantifications with mean values and SDs from three independent experiments of the binding assays are shown in the graph bar diagram. (D) Similar to B, except that in vitro–translated 35S-labeled Myc-tagged DmVps34 was incubated with GST or GST-Zda. The graph represents the mean value with SD from three independent experiments.