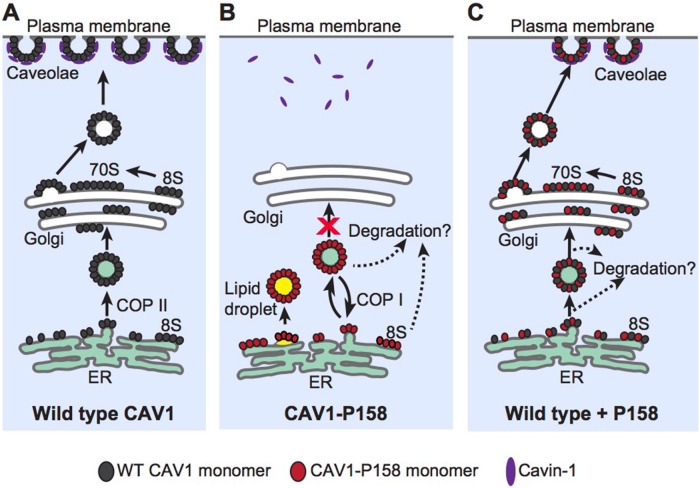
FIGURE 12:
Proposed model of CAV1-P158 trafficking in the absence or presence of WT CAV1. (A) In control cells, newly synthesized WT CAV1 forms 8S complexes and efficiently exits the ER. It is subsequently transported from the Golgi complex to the plasma membrane in the form of 70S complexes where it recruits cavin1 and forms caveolae. (For simplicity, other accessory proteins are not shown.) (B) Unlike WT CAV1, CAV1-P158 is incapable of supporting caveolae formation when expressed on its own due to the introduction of an ER retention signal by the frameshift mutation. Instead, it is retained in the ER and lipid droplets where it can also be targeted for degradation. (C) Coexpression of WT CAV1 and CAV1 P158 results in the formation of hybrid complexes of the two proteins. These hybrid complexes have at least two possible fates. A fraction of the hybrid complexes go on to form 8S and 70S complexes and are targeted to the cell surface where they form hybrid caveolae. These hybrid caveolae are apparently morphologically normal but are less detergent resistant than WT caveolae. A second fraction of hybrid complexes are unable to exit the ER and/or are rapidly degraded, thus decreasing overall CAV1 expression levels. What determines whether the hybrid WT/ P158 complexes ultimately reach the cell surface or are retained in the ER/degraded is unknown but is likely to reflect the relative ratio of WT and mutant CAV1 within a given complex.